 Supply Chain/Technology
Supply Chain/Technology
The rise of AI in radiology—and what the future holds

When it comes to the adoption of artificial intelligence (AI) in medicine, radiology is leading the charge. As of May 13, 2024, the US Food and Drug Administration (FDA) had approved nearly 900 AI- and machine learning (ML)-enabled devices, and the vast majority of them are in radiology. One example…
Ambulatory endoscopy management strategies keep patients, finances healthy
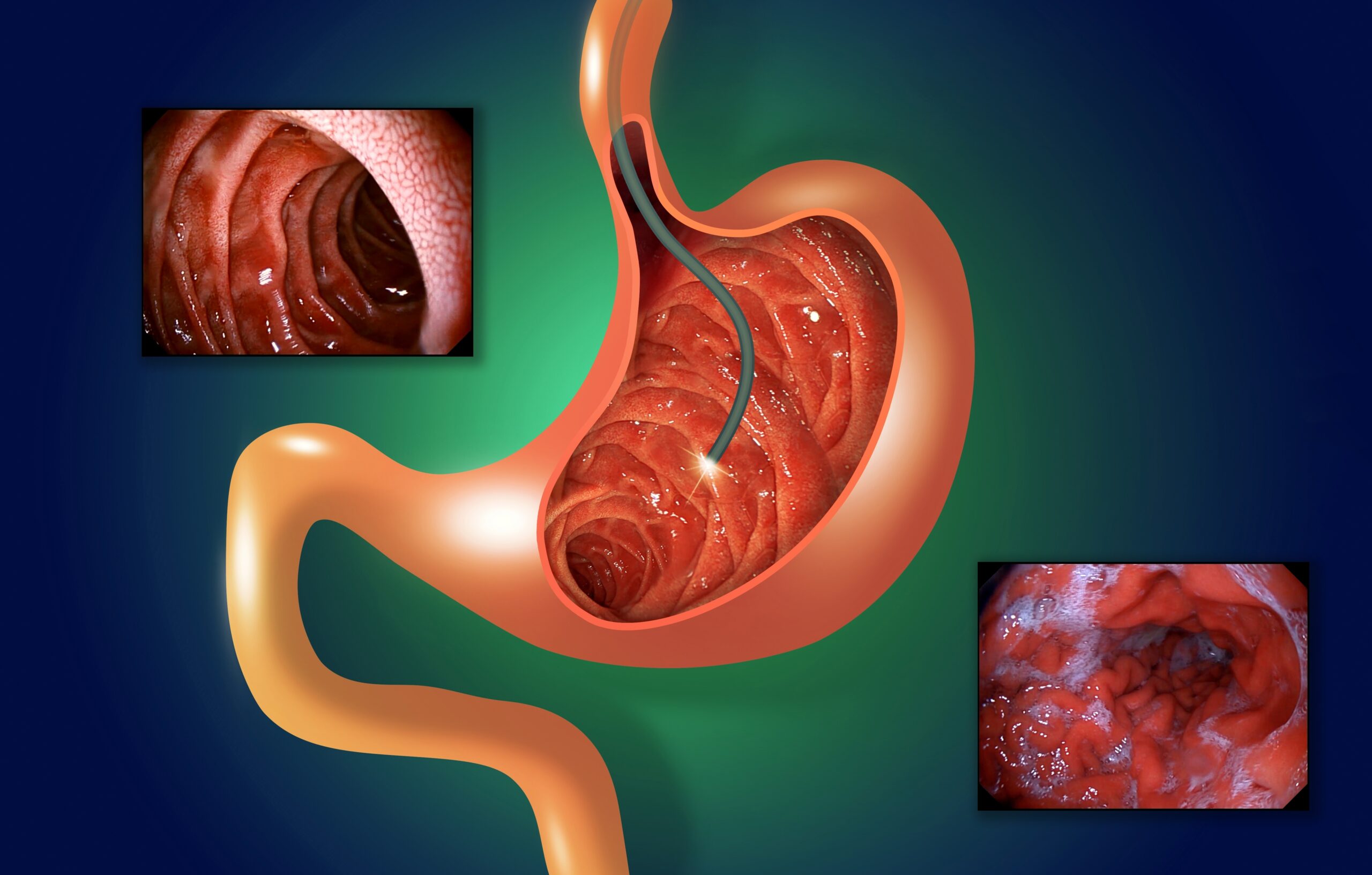
Gastrointestinal (GI) endoscopy is one of the most common procedures in the US. Performed more than 17.1 million times per year in inpatient and outpatient hospital settings as well as ambulatory surgery centers (ASCs), GI procedures account for 68% of all endoscopies, according to a May 2022 article in Digestive…
Rural hospitals contend with challenging opportunities

Rural hospitals in the US have been facing a prolonged, multifaceted crisis. The literature presents several reasons for why healthcare facilities in rural areas struggle, including shrinking budgets, rising chronic illness and public health issues like addiction and obesity, poor telehealth and broadband access, aging populations, deteriorating mental health, and…
Ketamine, other anesthetics show promise for depression, mental health treatment

Editor's Note The advance of ketamine and other anesthetics as depression treatments is spurring collaboration among anesthesiologists and psychiatrists for further advances in mental health treatment, according to an article in the June issue of Anesthesiology, the peer-reviewed journal of the American Society of Anesthesiologists. As the established experts in…
CMS to end Change Healthcare cyberattack assistance program

Editor's Note The Centers for Medicare & Medicaid Services (CMS) has announced assistance for providers affected by the Change Healthcare cyberattack ends next month. According to the June 17 announcement, payments under the Accelerated and Advance Payment (AAP) Program for the Change Healthcare/Optum Payment Disruption (CHOPD) will end July 12,…
Study: Immunotherapy offers significant benefits for dMMR colorectal cancer
Editor's Note A pair of immunotherapy drugs administered before surgery significantly diminished tumor size without serious safety concerns in patients with mismatch repair-deficient (dMMR) colorectal cancer, according to a study published in the New England Journal of Medicine. Healthline reported the news June 8. Constituting 10-15% of cases, dMMR cancer…
Study: Liver surgery safe for outpatient settings

Editor's Note Robotic liver surgery can be performed safely as an outpatient procedure, according to findings from the City of Hope cancer research organization in Duarte, California. According to a June 10 press release, the study analyzed data of 307 patients who underwent outpatient robotic liver surgery (defined as requiring…
Study: Bariatric surgery outperforms GLP-1 treatment, lifestyle modifications

Editor's Note Bariatric surgery provides longer-lasting, more effective weight loss than GLP-1 receptor agonists and lifestyle interventions, according to systematic reviews of medical literature from 2020 to 2024. Medical Xpress reported the news June 11. Presented at the American Society for Metabolic and Bariatric Surgery (ASMBS) 2024 Annual Scientific Meeting,…
AI, more pay help healthcare executives combat worsening nurse shortage

Editor's Note Healthcare executives expect the US nurse shortage to worsen, according to the 2024 Healthcare Executive Report from Incredible Health, a career marketplace for healthcare workers. Projected to reach 1 million by 2030, the shortage is exacerbating stress on current staff and compromising care quality, according to the company’s June…
Ascension announces full EHR restoration after cyberattack disruption

Editor's Note Ascension has restored electronic health records (EHRs) throughout all hospitals and clinics nationwide, according to a June 14 update from the St. Louis-based health system. "Clinical workflow in our hospitals and clinics will function similarly to the way it did prior to the ransomware attack," the statement reads,…

 Free Daily News
Free Daily News