 Supply Chain/Technology
Supply Chain/Technology
FDA issues Class 1 recalls for two ventilator models

Editor's Note Alarm failures and missing details in instructional materials prompted the US Food and Drug Administration (FDA) to issue class 1 recalls—the most severe category indicating risk of serious injury or death—for two different ventilator models on June 27. According to the report, failure in the Ventilator Inoperative alarm…
Post-CABG cardiac shockwave therapy shows promise in early study

Editor's Note Using a device they call a “space hairdryer,” researchers in Austria applied gentle shockwaves to regenerate heart tissue after coronary artery bypass graft surgery (CABG) in a study with potential implications for millions of patients, BBC News reported June 20. Researchers are now seeking larger trials, European regulatory…
Study finds no link between anesthesia dose, postop delirium

Editor's Note Higher doses of anesthesia did not affect risk of postoperative delirium in a study of more than 1,000 heart surgery patients, according to a June 10 United Press International (UPI) article on study findings published in JAMA. The research included 1,140 heart surgery patients, half of whom had…
AI outperforms radiologists in detecting clinically significant prostate cancer

Editor's Note In a recent study, an artificial intelligence (AI) system detected more clinically significant prostate cancers and fewer indolent cancers than human radiologists reading MRIs, MedPage today reported June 13. The MedPage report covers a study published in Lancet Oncology that, according to researchers, “provided evidence that AI systems,…
FBI, HHS issue healthcare cybersecurity warning

Editor's Note A June 24 advisory from the FBI and Department of Health and Human Services warns healthcare organizations about attempts to steal payments through phishing and social engineering tactics, according to a post from the American Hospital Association (AHA). The attackers target employee email accounts to access login information…
Kidney transplant performed on awake patient

Editor's Note In a first for Northwestern Medicine, surgeons performed a kidney transplant on an awake patient, CBS News reported June 24. John Nicholas, 28, of Chicago, experienced no pain during the May 24 procedure, in which he received an organ from a childhood friend. He was discharged the next…
Russian ransomware group threatens cybersecurity beyond London attack

Editor's Note Qilin, a ransomware group based in Russia, claimed responsibility for a cyberattack against pathology services provider Synnovis that paralyzed London Hospitals and is now requesting $50 million, Becker’s Health IT reported June 20. Citing a report from Bloomberg, the article notes that the attack disrupted services at London-based hospitals…
Change Healthcare issues notifications of patient data stolen in cyberattack

Editor's Note Change Healthcare has started to notify health care providers about patient data stolen in the February cyberattack and announced plans to mail affected individuals as well. A unit of UnitedHealth Group, the organization issued the update June 20. “CHC is providing this notice now to help individuals understand…
Ambulatory endoscopy management strategies keep patients, finances healthy
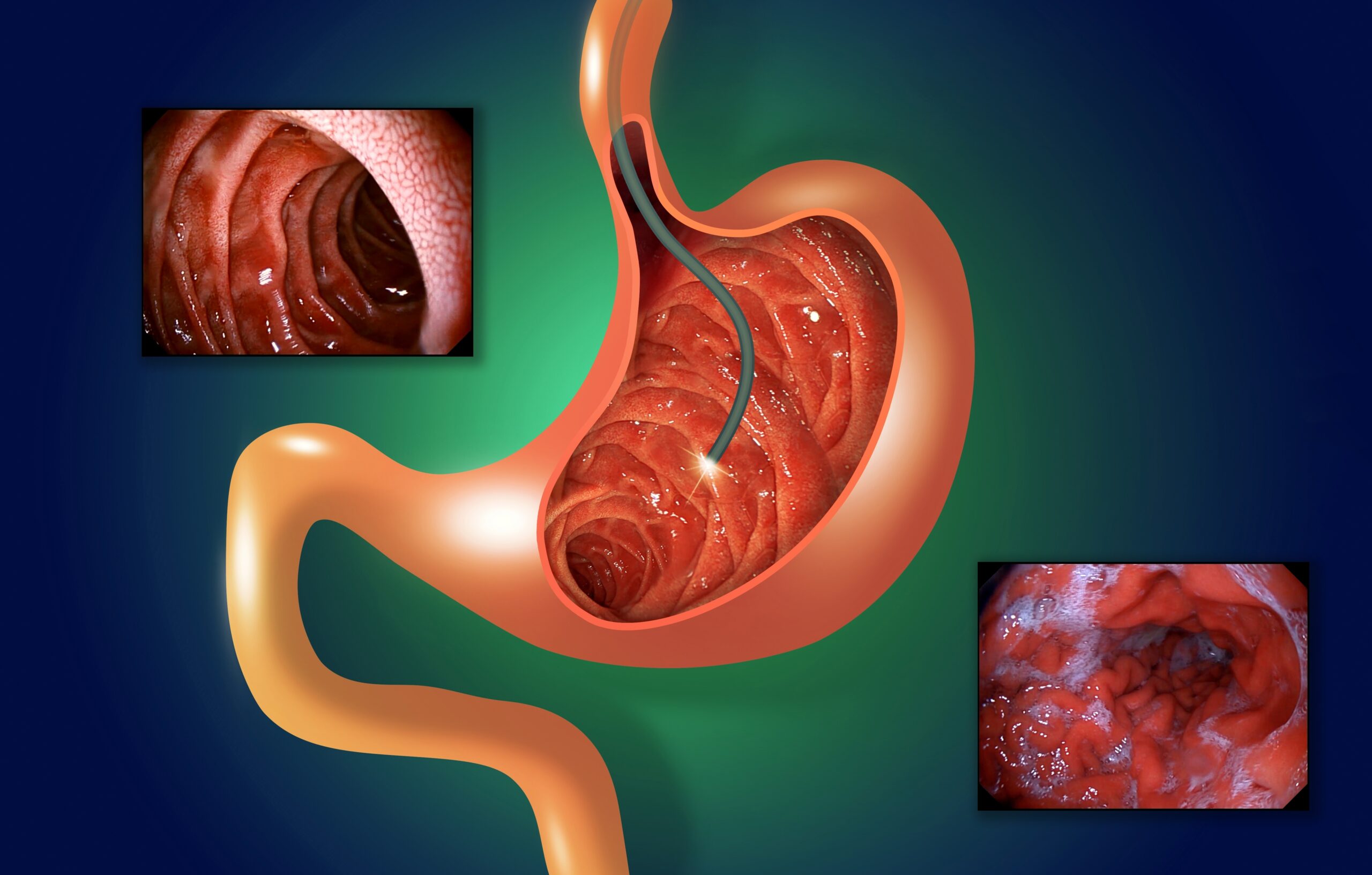
Gastrointestinal (GI) endoscopy is one of the most common procedures in the US. Performed more than 17.1 million times per year in inpatient and outpatient hospital settings as well as ambulatory surgery centers (ASCs), GI procedures account for 68% of all endoscopies, according to a May 2022 article in Digestive…
Rural hospitals contend with challenging opportunities

Rural hospitals in the US have been facing a prolonged, multifaceted crisis. The literature presents several reasons for why healthcare facilities in rural areas struggle, including shrinking budgets, rising chronic illness and public health issues like addiction and obesity, poor telehealth and broadband access, aging populations, deteriorating mental health, and…

 Free Daily News
Free Daily News