Offsite sterilization fuels onsite efficiency for lean ASCs

For many in the healthcare industry, imagining surgery without onsite sterile processing seems unthinkable. Then again, performing total joints in an ambulatory surgery center (ASC) was unthinkable 10 years ago. ASC sterile processing departments (SPDs) are generally not designed to handle the high volumes of instrument trays, vendor trays, and…
Water quality: 5 Ws and an H for sterile processing pros

Asking who, what, why, when, where, and how—otherwise known as the “5 Ws and an H”— is a time-tested way for writers and researchers to ensure comprehensive coverage of any topic. Here, we apply this framework from the perspective of sterile processing department (SPD) professionals seeking to start a water…
AAMI updates guidelines on radiation sterilization validation, single-use systems control

Editor's Note In a new guidance document for manufacturers of pharmaceuticals and biopharmaceuticals, The Association for the Advancement of Medical Instrumentation (AAMI) has released a new guidance document updating best practices for radiation sterilization validation and routine control of single-use systems. The document, AAMI CR513:2024; Guidance on radiation sterilization validation and…
Scaling standards from sterile processing department to clinic
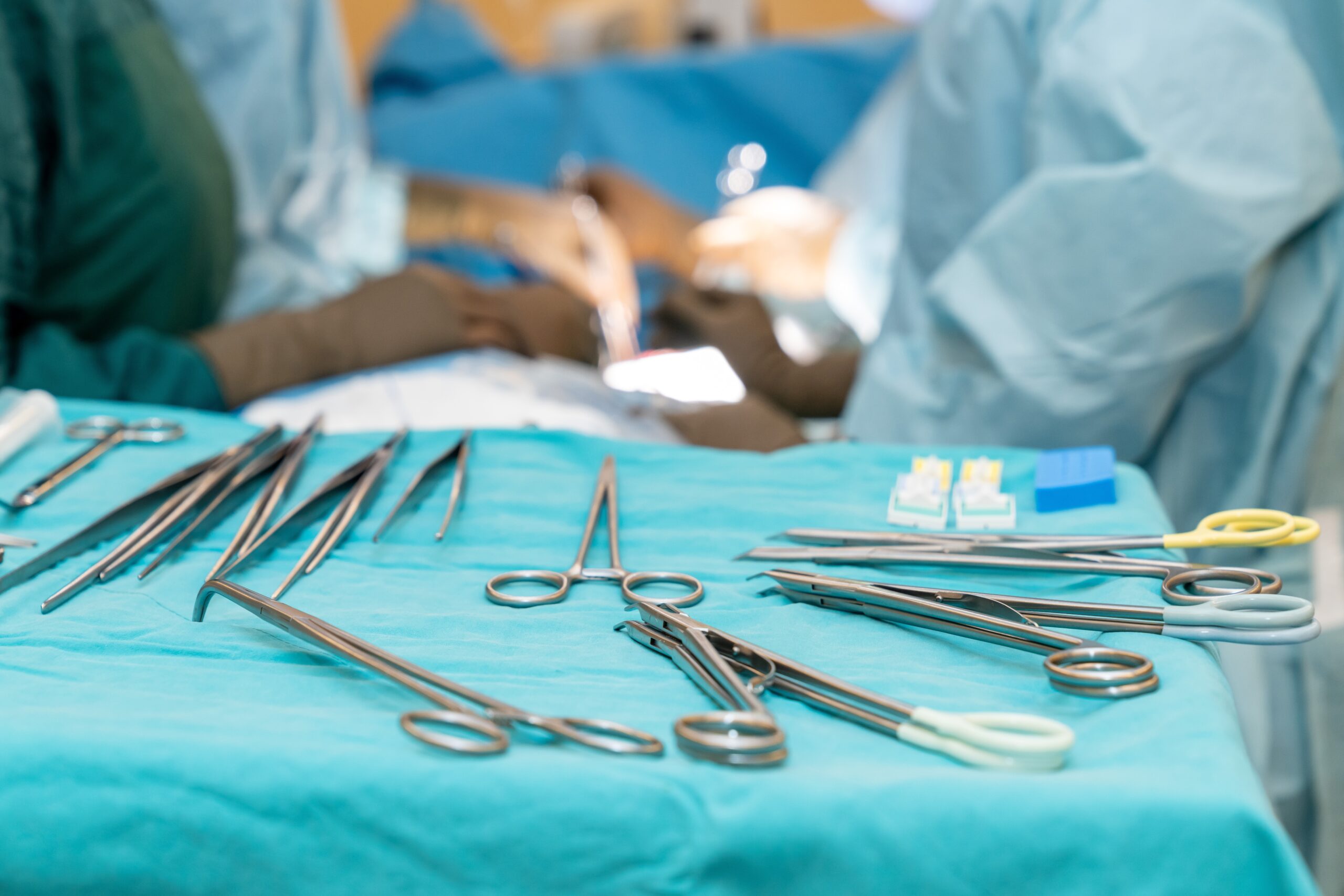
Reforming instrument reprocessing practices does not always end with the main sterile processing department (SPD). Holding clinics to the same standard adds to the challenge, whether they are associated with hospitals or operate independently. Nonetheless, standardization is just as essential to maintaining efficiency and quality standards. Whether a clinic is…
Common inspection points for surgical instrumentation
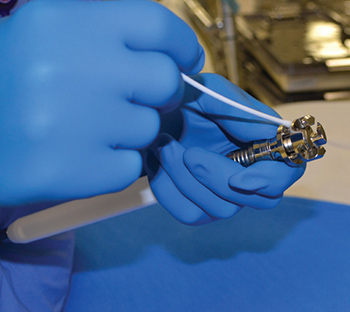
Inspecting surgical devices is a time-consuming process. However, diligently checking every instrument prior to sterilization is essential to ensuring safe, proper functioning. As the last people to see devices before they are used for patient care, sterile processing technicians must be thorough. Exterior surfaces should be inspected for flaws such…
AAMI guidance covers sterile processing of dilators, ultrasound probes

Editor's Note A new guidance document covering the entire process for the selection, labeling, and sterile processing of dilators and ultrasound probes is available from The Association for the Advancement of Medical Instrumentation (AAMI). Released April 17, AAMI TIR99:2024; Processing Of Dilators, Transesophageal And Ultrasound Probes In Health Care Facilities…
New EPA standards to reduce ethylene oxide emissions

Editor's Note New standards from The Environmental Protection Agency promise to cut nationwide emissions of ethylene oxide—employed to sterilize more than half of US medical devices—by more than 90 percent. According to a March 15 MedPage Today report, the aim is to reduce cancer risk among the 13 to 14…
Managing immediate use steam sterilization

Immediate use steam sterilization (IUSS, a standard steam sterilization cycle with little to no dry time) is considered safe for patient care when the processes recommended in Association for the Advancement of Medical Instrumentation (AAMI) standards and AORN guidelines are followed. IUSS is a valuable option in an emergency. Lack…
Keeping it sterile: Fundamentals of sterile storage

Using sterile items in surgery is a fundamental practice, not a rudimentary one that can or should be taken for granted. Put simply, using unsterile items can result in a patient infection. If sterile storage conditions are not appropriate, the items can become contaminated. Such contamination may go undetected, rendering…
New borescope training model improves SPD personnel knowledge, complex skills
Editor's Note This pilot study by epidemiologist Cori L. Ofstead, MSPH, and colleagues finds that a new borescope training model improves sterile processing department (SPD) personnel mastery and retention of complex skills. A total of nine certified SPD personnel were involved in the study. Training focused on borescope visual inspection…

 Free Daily News
Free Daily News