 Supply Chain/Technology
Supply Chain/Technology
FDA recalls affect surgical staplers, OR procedure kits, ultrasound systems used in hospitals

Editor's Note The Food and Drug Administration (FDA) has announced three new recalls between October 14 and 16 that may affect OR inventory and perioperative workflows across multiple service lines. Each recall targets a commonly used product in surgical or imaging settings, prompting leaders to review supplies and coordinate with…
AI model grades nursing procedures with near-human precision

Editor's Note A new study shows video-language models (VLMs) can accurately evaluate nursing skills and generate meaningful feedback, potentially transforming how future nurses are trained and assessed, Cornell University October 6 reports. The study describes the first framework to apply VLMs to automated nursing competency evaluation. According to the article,…
Integrating robotics for outpatient surgery: What ASC leaders need to know
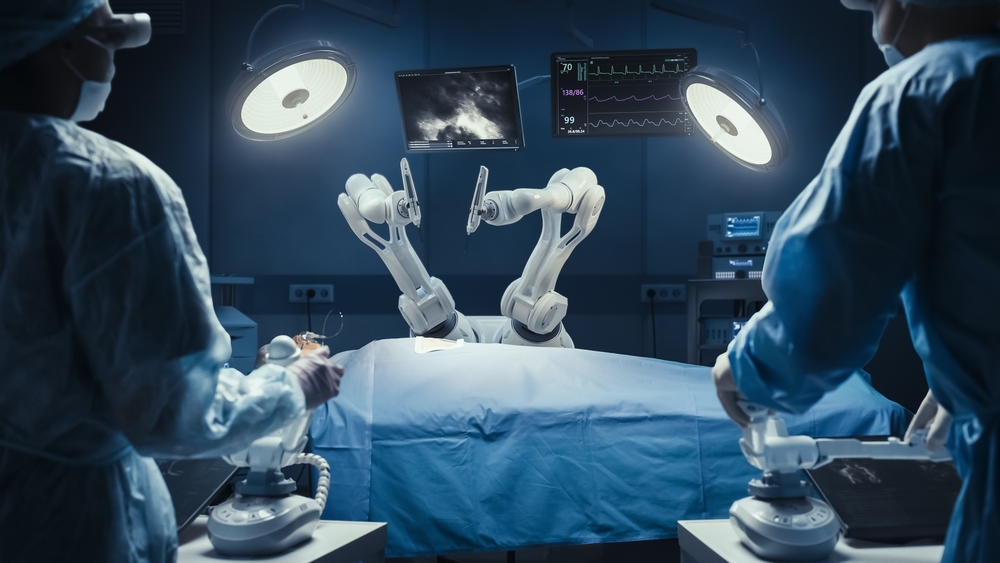
Robotic surgery has moved from cutting-edge to commonplace. The question is no longer whether to use robotics but when to introduce it and how to ensure adoption is efficient, affordable, and seamless for surgical teams. Ambulatory surgery centers (ASCs) are increasingly adding robotics to their service lines, driven by the…
Telehealth in limbo: Providers split on continuing Medicare services during shutdown

Editor's Note Telehealth providers are divided over whether to continue serving Medicare patients after reimbursement expired alongside the federal government shutdown, Modern Healthcare October 9 reports. The impasse has forced organizations to weigh patient access against financial risk, with many issuing advance beneficiary notices warning patients they may be responsible…
Sustainable surgery can cut costs, reduce emissions, improve care, review finds

Editor's Note Surgical teams can dramatically reduce healthcare’s carbon footprint through waste reduction, energy efficiency, and smarter procurement, Cureus October 7 reports. The review’s authors describe surgery as both a major environmental challenge and a key opportunity for hospitals to align climate responsibility with clinical and financial goals. Healthcare contributes…
How digital tools help nurse leaders retain expertise, stabilize staffing, sustain efficiency

Perioperative leaders face a workforce transition unlike any in recent memory. By 2030, all baby boomers will have reached retirement age, and many veteran perioperative nurses are already exiting, taking with them decades of institutional knowledge. At the same time, the expectations of today’s workforce have shifted, placing greater value…
Federal pushback on CHAI exposes rifts over who should set healthcare AI rules

Editor's Note Federal officials’ public rebuke of the Coalition for Health AI (CHAI) highlights mounting tensions over who should shape guardrails for artificial intelligence (AI) in healthcare, Modern Healthcare October 10 reports. As hospitals accelerate AI adoption, industry leaders, regulators, and developers are clashing over how to ensure the technology’s…
FDA issues Class I alert for Abiomed Impella heart device over cybersecurity risks

Editor's Note The Food and Drug Administration (FDA) on October 10 classified a cybersecurity correction involving Abiomed’s Automated Impella Controller as a Class I recall, the most serious type, according to the FDA Medical Device Recalls and Early Alerts database. While devices are not being removed from clinical settings, the…
FDA accelerates medical device safety alerts to reach public within days

Editor's Note The Food and Drug Administration (FDA) has expanded its early alert recall program to include all medical devices, speeding up how quickly the public learns about high-risk safety issues, Modern Healthcare October 9 reports. The initiative allows the FDA to post early alerts within days of manufacturers notifying…
Considerations for external transportation of processed medical devices

The centralization of medical device processing to one facility is becoming more prevalent. Centralizing sterile processing activities reduces expenses while concentrating expertise. However, this also introduces new concerns. When sterile processing is located within the same building where instrumentation is used, transport occurs over smooth floors in a controlled environment…

 Free Daily News
Free Daily News