 Regulations/Legal
Regulations/Legal
How competency assessment could extend beyond licensing
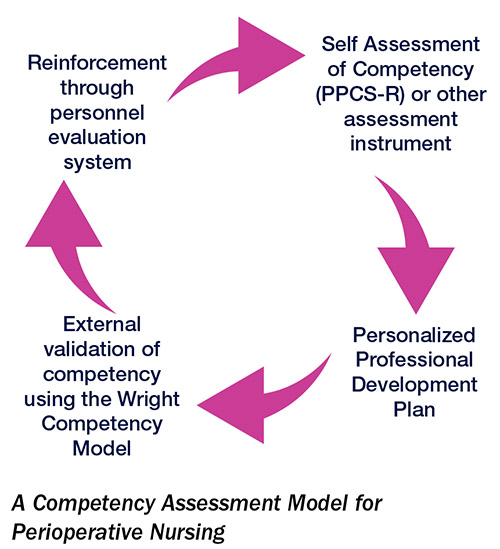
Competency assessment in perioperative nursing—and American healthcare in general—is a story of unrealized potential. Particularly in the wake of the pandemic, staffing shortfalls and financial pressures have made focusing on staff development difficult for nurse leaders. Nonetheless, the argument for investing more in professional development and competency has never been…
Scaling standards from sterile processing department to clinic
Reforming instrument reprocessing practices does not always end with the main sterile processing department (SPD). Holding clinics to the same standard adds to the challenge, whether they are associated with hospitals or operate independently. Nonetheless, standardization is just as essential to maintaining efficiency and quality standards. Whether a clinic is…
CMS to end Change Healthcare cyberattack assistance program

Editor's Note The Centers for Medicare & Medicaid Services (CMS) has announced assistance for providers affected by the Change Healthcare cyberattack ends next month. According to the June 17 announcement, payments under the Accelerated and Advance Payment (AAP) Program for the Change Healthcare/Optum Payment Disruption (CHOPD) will end July 12,…
Understanding bipartisan push for site-neutral Medicare payments aiming to curb healthcare costs, consolidation

Editor's Note Amid growing concerns over healthcare spending and affordability, there is bipartisan interest in aligning Medicare payments for outpatient services across various care settings through "site-neutral" payments, KFF June 14 reports. As a June 2023 Modern Healthcare article explains, last year Congress reviewed legislation to expand site-neutral payment policies,…
State legislative challenges impacting ASCs: Updates from North Carolina, California, Massachusetts, South Carolina

Editor's Note In a recent ASCA Audio Update, Charlie Leonard, public relations and public affairs consultant, spoke with Steven Obrech, associate director of government affairs at ASCA, to discuss various legislative issues affecting ambulatory surgery centers (ASCs). This discussion showcased ongoing legislative challenges and efforts to support ASC operations at…
Ascension announces full EHR restoration after cyberattack disruption

Editor's Note Ascension has restored electronic health records (EHRs) throughout all hospitals and clinics nationwide, according to a June 14 update from the St. Louis-based health system. "Clinical workflow in our hospitals and clinics will function similarly to the way it did prior to the ransomware attack," the statement reads,…
Updated ethylene oxide sterilizer standard covers latest technology

Editor's Note The Association for the Advancement of Medical Instrumentation (AAMI) has updated its standard on ethylene oxide (EO) sterilizers for healthcare facilities, according to a June 12 press release. The first update in two decades, the fourth edition ANSI/AAMI ST24:2024 covers labeling, safety, performance, and testing requirements for general-purpose…
Patient files stolen in Ascension cyberattack

Editor's Note Personal patient data could have been compromised in the May 8 cyberattack on Ascension, according to the latest update from the St. Louis-based healthcare system. Posted June 12, the update reveals that attackers accessed files from seven out of 25,000 file servers used for routine tasks, potentially containing…
Health systems focus on long-term growth, outpatient care amid construction challenges

Editor's Note Health systems are increasingly focusing on construction projects aimed at long-term growth and outpatient care, often renovating existing facilities, Modern Healthcare June 10 reports. According to Modern Healthcare's 2024 Construction and Design Survey, 60% of respondents see growth in the healthcare construction industry despite inflation, high material costs,…
Announcing the ASC administrators CASC training course

Editor's Note The OR Manager Conference is proud to announce it will offer a 1-day training course outlining core areas of study for the Certified Administrator Surgery Center (CASC) exam to earn the CASC credential. This training is ideal for those responsible for the operations of running an ambulatory surgery…

 Free Daily News
Free Daily News