
Editor's Note Rebuilding trust and redefining teamwork between the OR and sterile processing department (SPD) turned a near-failure into a high-functioning system at UCLA Health, according to Ronald Perez, JD, MSN, RN, NEA-BC, CNOR, executive director of perioperative services, and Jasmine Briones, MSN, RN, CNOR, director of perioperative services. What…

Editor's Note Sterile processing departments (SPDs) face chronic staffing shortages and underinvestment that put surgical patients at risk, according to a Surgical Directions September 18 report. It emphasizes that sterile processing technicians, who decontaminate, inspect, and sterilize every surgical instrument, remain under-recognized despite their central role in surgical safety. Per…
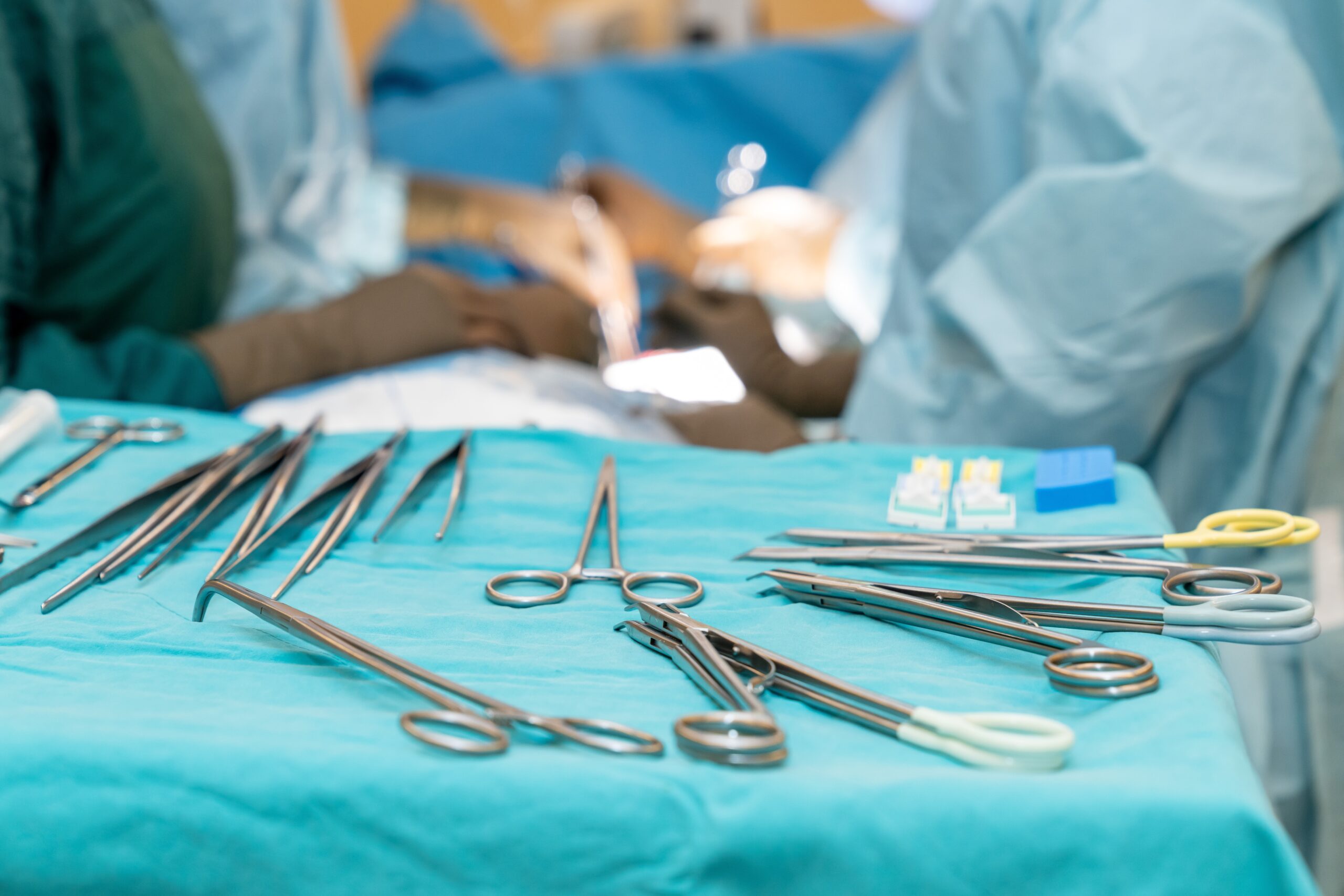
Once limited to hospital inpatient settings, total joint surgery is increasingly common at ambulatory surgery centers (ASCs) across the US. What is not so common is performing these complex procedures without the benefit of an onsite sterile processing department (SPD). And yet, that is exactly what we have accomplished at…

Editor’s Note: This page is a companion piece to the main article, Centralized sterile processing cuts costs, complexity for four ASCs. Implementing offsite sterilization is a major project. At Total Joint Specialists, our journey began gradually, growing in scope over time as the team became comfortable with the process…
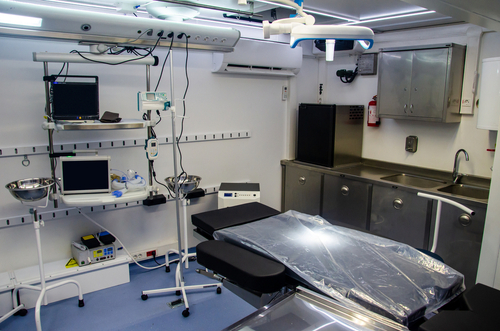
Imagine an innovative, safe, and highly efficient OR not confined by walls but on wheels—crossing rugged terrains, bustling cities, and disaster-stricken areas to deliver life-saving surgical care in underserved areas. That is the premise and promise of mobile ORs. They are not just mobile units. With some of the technological…

Editor's Note New and revised sterilization and reprocessing standards are reshaping the landscape for sterile processing departments, placing greater emphasis on chemical modalities, device-specific protocols, and system-wide quality management, according to the Healthcare Purchasing News May 27 update on compliance and standards. Among the most significant developments is the overhaul…

Preventing infection from contaminated surgical tools requires attention to every link in a complex chain of processes, from point-of-use pretreatment in the OR to the moment the freshly disinfected or sterilized item arrives at the next patient’s bedside. For those on the front lines, manufacturers’ written instructions for use (IFUs)…

Patient safety and regulatory compliance demand thoroughly vetting those tasked with preventing healthcare-associated infections. But which certification is the right fit? Those who are new to infection control have a wide range of options for verifying their newly acquired expertise. More seasoned infection preventionists (IPs) also have a number of…

Editor's Note The Association for the Advancement of Medical Instrumentation (AAMI) has released the updated AAMI TIR17:2024; Compatibility of Materials Subject to Sterilization, its first revision since 2017. This guidance provides essential information for medical device manufacturers, designers, and sterilization professionals on how sterilization methods impact materials and packaging. Updates…

Reliable and robust enough for daily use on most medical devices, steam is the most common sterilant in healthcare facilities. However, using steam properly requires a balancing act. For example, too much moisture can lead to wet packs, while steam that is too dry might not be sufficient to achieve…