 Supply Chain/Technology
Supply Chain/Technology
FDA: Class I recall of Insulet Omnipod DASH PDM
Editor's Note The Food and Drug Administration (FDA) on November 16 identified the recall by Insulet of its Omnipod DASH Insulin Management System Personal Diabetes Manager (PDM) as Class I, the most serious. The recall was initiated because of PDM battery issues, including: Battery swelling Fluid leakage from the battery…
Augmented and virtual reality bring high-tech efficiency to the OR

COVID-19 was a catalyst that helped accelerate innovation in healthcare by a decade, says Jay Banerjee, chief operating officer and cofounder of ImmersiveTouch, a virtual reality (VR) surgery company in Chicago. “Many things that would have taken 20 to 30 years happened sooner,” Banerjee says. “Telemedicine; electronic health records; remote…
Editorial

After writing my latest article on surgical robots (Operationalizing a robotics program for evenings, weekends, pp 13–15, 19) for this issue of OR Manager, I reminisced on how the surgical robot has evolved and how my interest in surgical robots began. Reminiscing about robots It all began with the…
Operationalizing a robotics program for evenings, weekends
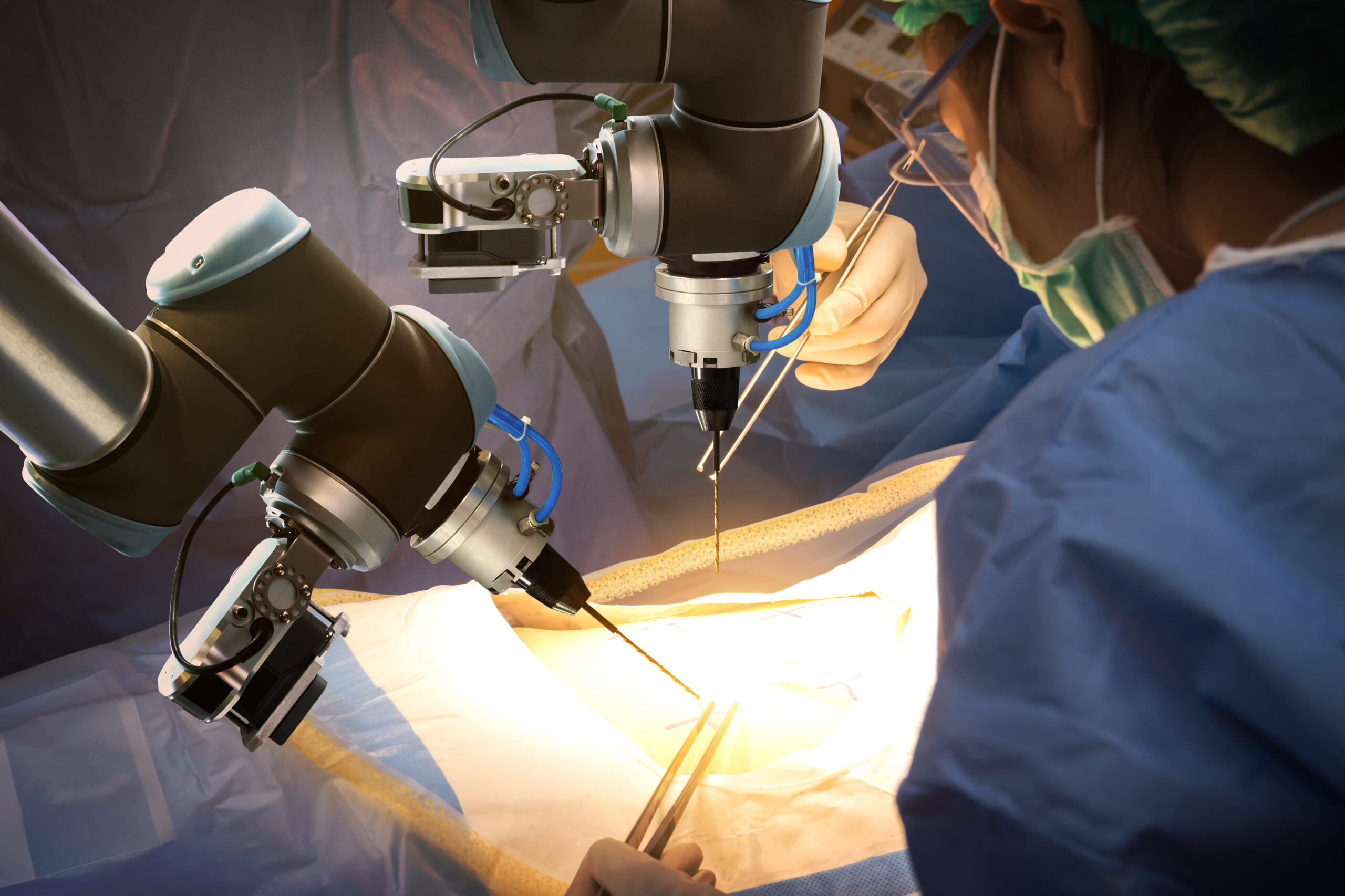
The St Elizabeth Healthcare organization in northern Kentucky has eight facilities, including two surgery centers. The flagship hospital on the Edgewood campus has 534 beds and 22 ORs and typically performs some 14,000 surgical procedures per year. Robots help manage the volume. The Edgewood, Florence, and Ft Thomas hospitals have…
State of natural language processing in surgery: Part 1

Imagine this: As a nurse in the OR talks with a patient, a computer listens in, recording the nurse’s notes so that data are added automatically to be retrieved later. The surgeon and anesthesiologist also interact with the patient without the burden of manual data entry, and the insurance company…
Revised AORN Flexible Endoscope Processing Guideline

On September 15, 2022, AORN released its updated Guideline for Processing Flexible Endoscopes. Over the past several years, there has been significant research on flexible endoscope processing—research that met the stringent requirements and has been included in this revised guideline by AORN’s lead author and team. Endoscope processing begins with…
Casey Orth-Nebitt: Perioperative Nurses Week Interview

Today’s chat is with Casey Orth-Nebitt, MSN, RN, CNOR, Director of Surgery, Buena Vista Regional Medical Center, Storm Lake, Iowa. Q: What is something that went right at your facility and/or that you are thankful for this year? I'm always thankful for my staff, who have remained loyal, hardworking, and…
Supply chain expert warns that drug shortages will persist
Editor's Note Vials of the chemotherapy drug fludarabine could be purchased at a whole sale price of $110 last year—today, it is being sold for a much higher price, Fierce Pharma November 1 reports. One company, Areva Pharmeceuticals, increased the price to $2,736, Stat News reported on October 27. This…
Florida health system improved patient care by enacting SDOH changes to EHR
Editor's Note Memorial Healthcare System in Hollywood, Florida, via an initiative spearheaded by Jennifer Goldman, DO, chief of Memorial Primary Care, began including social determinants of health (SDOH) data in its electronic health record (EHR), “where doctors can see and act of them”—and that is improving patient care, HealthLeaders November…
Study: 79% of patients felt in control of their health with virtual care
Editor's Note According to a November 1 news release from Business Wire, a study done by Elevance Health shows that to 79% of patients surveyed, virtual primary care, or telehealth, has allowed them to take charge of their health, Becker’s Hospital Review November 1 reports. The study was conducted online…

 Free Daily News
Free Daily News