 Supply Chain/Technology
Supply Chain/Technology
Navigating the ‘tripledemic’: Flu, RSV, and COVID-19 in the OR

The healthcare sector has begun grappling with a repeat challenge this fall and winter: the simultaneous surge of three significant respiratory diseases—flu, respiratory syncytial virus (RSV), and COVID-19 among both children and adults. When these diseases collide, they constitute what experts have termed a “tripledemic,” which poses a significant strain…
Optimizing productivity measurement in the ASC

Takeaways Clinical hours per case is a simple, yet effective, productivity metric. Analysis of productivity measures can include benchmarking against external and internal data. A positive work culture will improve staff productivity. Productivity is a foundational concept for leaders of ambulatory surgery centers (ASCs). “Measuring productivity is essential to be…
Role of robotics coordinators in standardization, efficiency
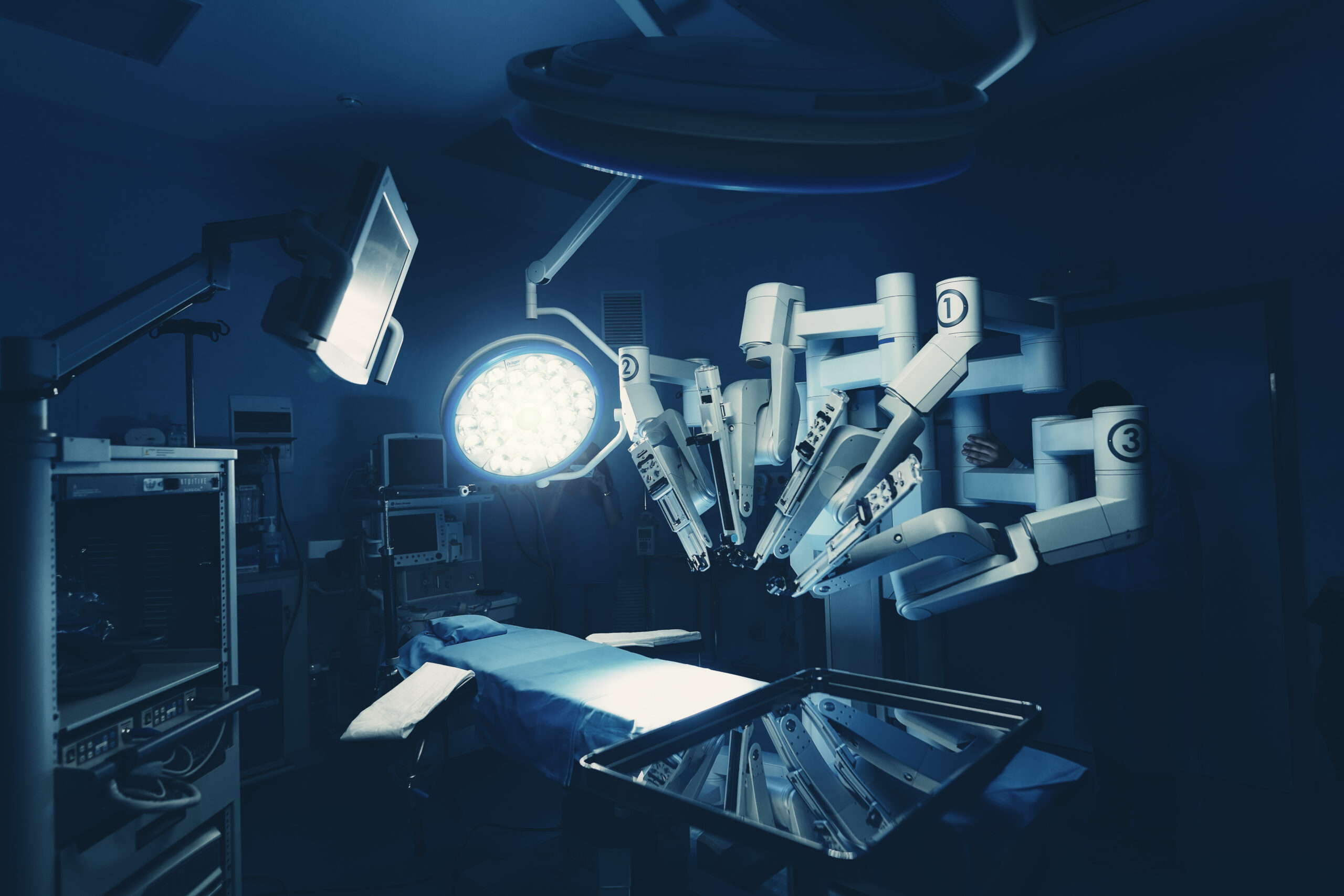
The importance of a dedicated robotics coordinator’s role in an efficient da Vinci robotics program cannot be overstated. “You need someone to take ownership of the program, set up training for staff and surgeons, and coordinate ongoing development,” says June Hill, BSN, RN, CNOR, clinical director of robotics, Inova Health…
How health systems are using tech tools to make ORs more efficient

Most hospitals agree that they can better harness the data they are collecting from registration through discharge in order to streamline patient turnovers, reduce wait times, and improve patient and staff satisfaction, but there is still uncertainty about how best to turn massive quantities of data into better patient outcomes.…
Research study assessing accuracy of ChatGPT in medical diagnoses
Editor's Note A May 2023 study shows that ChatGPT–a large language model artificial intelligence chatbot–can make accurate diagnoses and care management decisions but is less adept at differential diagnosis. The findings were published in the Journal of Medical Internet Research. The research team from Mass General Brigham inputted all 36…
Drug shortages are getting worse nationwide
Editor's Note Across the US, drug shortages are on the rise, including for chemotherapy drugs, antibiotics, and weight-loss drugs, PBS News November 5 reports. This trend is worrisome for many reasons, experts say, with the top-pressing concern being high-risk patients having to switch to less effective or more aggressive regimens…
New surgical implant allows Parkinson’s patient to walk again
Editor's Note A patient with Parkinson’s disease was able to walk normally again thanks to a surgical implant of an experimental spinal cord neuroprosthesis. The findings were published in the journal Nature on November 6 under the article title, "A spinal cord neuroprosthesis for locomotor deficits due to Parkinson’s disease." …
'Gig work' on the rise among nurses
Editor's Note Seeking higher pay and greater flexibility, many nurses are turning to gig work, MedicalXpress November 8 reports. The article defines gig work as “picking up individual shifts on an app as an alternative to months-long contracts or direct employment by a hospital.” Also according to the article: 100,000…
New cybersecurity toolkit provides guidance to healthcare sector
Editor's Note The Cybersecurity and Infrastructure Security Agency (CISA) and the Department of Health and Human Services (HHS) on October 26 announced a new cybersecurity-focused toolkit with resources for healthcare organizations and the public health sector. The two agencies have been working with the Health Sector Coordinating Council (HSCC) Cybersecurity…
ECRI: New survey shows medication, supply shortages are hurting patients
Editor's Note A new survey from ECRI finds that shortages of medications and medical supplies and equipment are harming patients, the nonprofit organization reported in an October 13 news release. ECRI surveyed nearly 200 pharmacists, pharmacy technicians, procurement specialists, physicians, and nurses in July 2023. Following are some takeaways from…

 Free Daily News
Free Daily News