 Supply Chain/Technology
Supply Chain/Technology
Study: UV-C light effectively disinfects non-sterile, high-touch surfaces

Editor's Note Although many studies have focused on infection transmission within the operating room, authors of research published in the March issue of the Journal of Infection Control focused their study of UV-C light disinfection on non-sterile hubs of patient care—in this case, high-touch surfaces within an academic endoscopy unit.…
Forced-air device outperforms standard endoscope drying practices, study shows

Editor's Note Authors of a recent study evaluating the effectiveness of a forced-air drying system for endoscopes argue that the results reinforce the need to re-evaluate standard drying practices. Findings were published February 24 in the American Journal of Infection Control. Wet environments resulting from inadequate drying practices can result…
Swifty’s singing keeps brain surgery on track

Editor's Note Staying awake during brain surgery to sing Taylor Swift songs helped ensure the best possible outcome for Selena Campione, a 36-year-old teacher from Stanhope, New Jersey who recently had a tumor removed at Jersey Shore University Medical Center. As detailed in a March 21 report from People, neurooncologist…
Procedural sedation analgesia considerations for ASC leaders
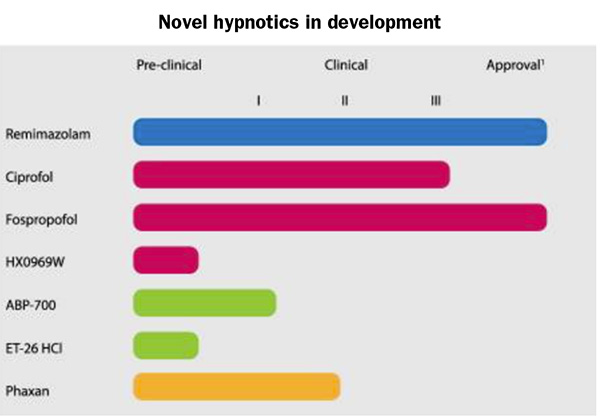
The promise of quicker recovery and fewer complications from sedation, anesthesia, and pain management have drawn clinicians and patients alike to procedures performed in ambulatory surgery centers (ASCs) and other outpatient settings. However, sedation, anesthesia, and analgesia add their own risks to those of the procedure itself. Understanding the latest…
Editorial: OR Business Management Conference tackled financial challenges, technological innovations

This past February, the OR Business Management Conference, held in Phoenix, Arizona, brought together business managers of all walks of life. The intimate event was once again a symbiotic blend between financial- and business-centric education and high-quality networking. The education offered for seasoned and new business leaders alike, perhaps more…
Surgical scrub evolution and the future of smart medical attire

For surgeons and other medical professionals, what to wear to work is more than just an afterthought. Over the decades, surgical scrubs have undergone a significant transformation, evolving from simple, functional garments to sophisticated attire that prioritizes both comfort and infection control. They are not merely clothing but a vital…
How gamification improves OR training, outcomes
Takeaways From mobile phone apps to immersive virtual reality surgery experiences, digital tools make healthcare training more interactive, more engaging, and more fun for everyone from nurses to surgeons. Modern procedures—including minimally invasive techniques and robotic surgery—require 50 to 100 cases for a surgeon to reach a safe proficiency level…
AI's pivotal role in transforming OR efficiency, now and in the future

Next-Gen Disruptors: Technologies Transforming the OR Installment #1, presented by LeanTaaS Surgical procedures are necessary steps in patient journeys—no one wants to have surgery, but most people will need it at some point for care continuity and better quality of life. To best support positive outcomes, optimal recovery periods, and…
Johnson & Johnson, NVIDIA promote artificial intelligence for the OR

Editor's Note Collaboration between Johnson & Johnson and Nvidia could soon enable surgeons to automate documentation by using artificial intelligence (AI) to scan video of procedures. CNBC reported the news March 18. Surgical video scans are just one possible application of the collaboration, with the report noting that “J&J’s MedTech…
Surgeons complete first successful animal-to-human kidney transplant
Editor's Note A 62-year-old man in Weymouth, Massachusetts is reportedly recovering well several days after receiving the first kidney to be transplanted into a living person. As noted in a March 21 report in the Boston Globe, the operation at Massachusetts General Hospital marked a new milestone in the effort…

 Free Daily News
Free Daily News