 Rules and Regulations
Rules and Regulations
Improving underrepresented patient participation in clinical trials: It matters who makes the request
Editor's Note In this investigative study done by the Boston Medical Center (BMC), patients were shown more likely to agree to participate in clinical studies when approached by research staff of the same race or ethnicity as them. The findings were published in JAMA Ophthalmology on October 19. The study…
New agreement between HHS, Pfizer to ensure access to Paxlovid
Editor's Note The US Department of Health and Human Services (HHS) and Pfizer have announced an agreement to extend patient access to the COVID-19 drug Paxlovid, Healthcare Purchasing News October 16 reports. According to the article, Pfizer is readying the drug for more availability in the commercial market in November…
Smaller volume blood draw tubes can prevent excess blood loss in sickest patients
Editor's Note Using a tube that collects about half the blood of a standard tube will still provide enough blood for a lab test while reducing transfusions for critically ill patients, a new investigative study published by JAMA Network reports. The study, titled "Small-Volume Blood Collection Tubes to Reduce Transfusions…
New study shows how initial exposure influences immune responses to COVID-19 variants
Editor's Note A new study suggests that someone's initial exposure to a specific COVID-19 variant shows some influence to their immune response to subsequent variants of SARS-CoV-2, the virus responsible for COVID-19, the University of Cambridge October 6 reports. The research, published by Science on October 6 and titled "Mapping…
Study: Routine ER screening catches undiagnosed type 2 diabetes, prediabetes
Editor's Note Early symptoms of type 2 diabetes often go undetected, and late detection can lead to long-term complications, including heart disease, nerve damage, and retinopathy. Screening for type 2 diabetes in the emergency department could reveal thousands of previously undiagnosed cases each year, EurekAlert! October 3 reports. These findings…
Postpandemic applicability, usage of UV disinfection technology

The COVID-19 pandemic highlighted the importance of proper disinfection to manage and contain the spread of the disease. During the pandemic, ultraviolet (UV) disinfection technology played an important role in safeguarding public health and combatting COVID-19. Various uses of UV technology have become available on the market and can be…
Minimizing wasted 'In-OR' minutes at start of day at a pediatric AMC
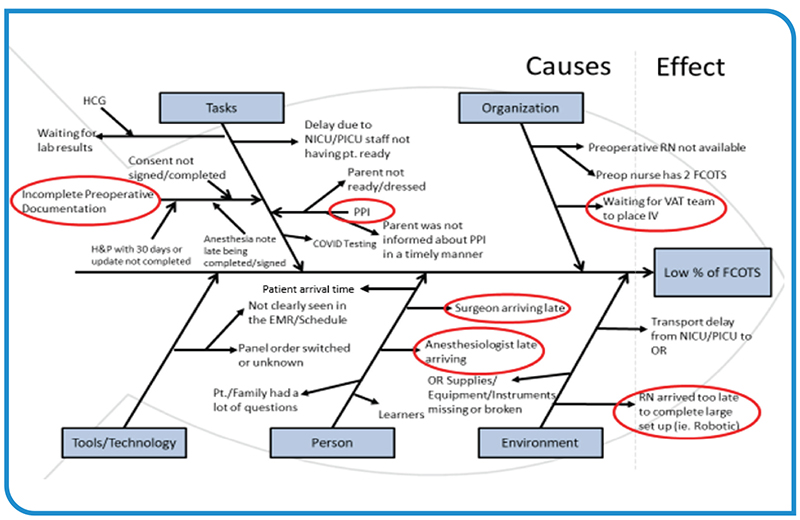
The OR is the financial motor of a hospital. As such, inefficiencies in this space adversely impact patient safety as well as the financial stability of the hospital and/or healthcare system. The first case on-time starts (FCOTS) metric is one of the primary benchmarks for assessing OR efficiency. The following…
Assistance program leads to major drop in ER visits, costs for immigrants
Editor's Note A study from economists and public health officials in the September 2023 issue of the journal American Economic Review: Insights found that when undocumented immigrants were provided assistance to visit primary care doctors via a pilot program, it resulted in a 21% drop in emergency room (ER) use.…
ASC luncheon: Quality Measure Reporting Updates for ASCs
Editor’s Note In this luncheon presentation, OR Manager Conference attendees delved into the Centers for Medicare & Medicaid Services Quality Measure Reporting Program for ambulatory surgery centers (ASCs). Gina Throneberry, MBA, RN, CASC, CNOR, director of education and clinical affairs at the Ambulatory Surgery Center Association (ASCA), shared which quality…
New CMS rule focuses on postop opioids in outpatient settings
Editor's Note The Non-Opioids Prevent Addiction in the Nation (NOPAIN) Act, which is set to take effect in 2025, will set up a separate Centers for Medicare and Medicaid Services (CMS) payment for certain nonopioid pain management techniques in outpatient and ambulatory surgery center (ASC) settings, the September 12 Becker’s…

 Free Daily News
Free Daily News