Proper packaging ensures integrity of sterilization
Sterilization packaging is considered a medical device and thus it must undergo validation testing before being used in healthcare facilities. To ensure proper packaging that will maintain the integrity of sterilization, consult the packaging manufacturers’ instructions for use (IFU) and follow the sterilization standards and guidelines from the Association for the Advancement of Medical Instrumentation (AAMI) and AORN.
There are basically three types of sterilization packages: flat wrappers, sterilization containers, and paper-plastic peel pouches. This article will discuss standards for sterilization packaging and sterilization packaging requirements for packaging manufacturers issued by the Food and Drug Administration (FDA).
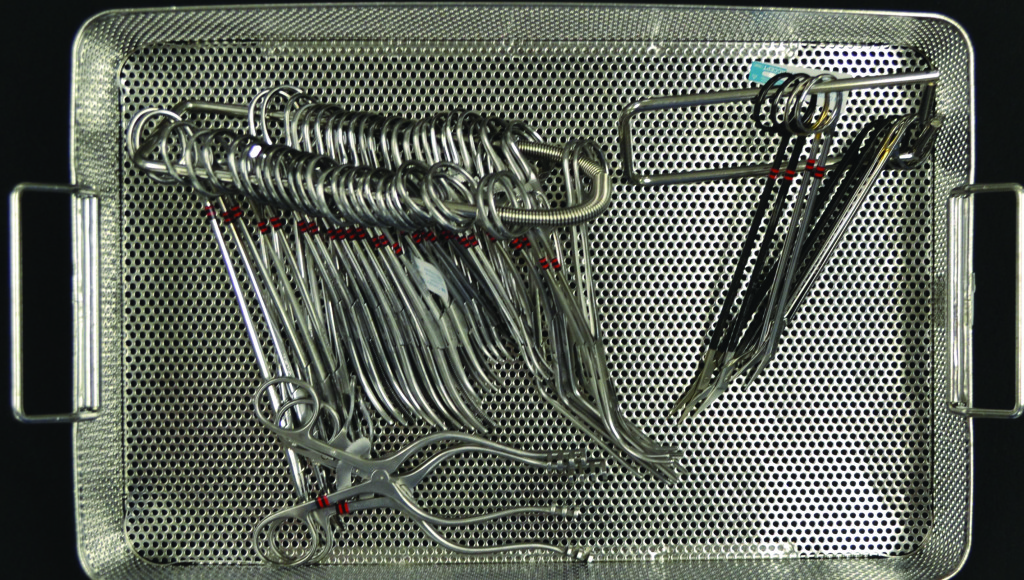
Chemical indictors are placed in the area of the instrument set that is least accessible to the sterilant. Photo courtesy of Susan Klacik. Used with permission.
Sterilization dating
Contamination of sterile packaging is considered an “event” that can result from a tear, dropped package, wet package, storing the package in a contaminated area, or discovering that a package is already open. Sterile storage is an important factor in event-related dating, which is why the temperature, humidity, and airflow of a storage area must be monitored and documented.
Sterile storage areas must be clean. The labeling of sterilization packaging may include sterility maintenance testing required by the FDA. This means the packaging manufacturer must perform testing that demonstrates the package can sit in a healthcare-simulated environment for a specific amount of time.
Healthcare facilities should follow AAMI and AORN best practices, both of which recommend event-related sterilization dating. Factors that affect the sterility of a package are packaging quality, handling, transport, and storage. A healthcare facility should have written policies and procedures that indicate how shelf life is determined and how it is identified on the package. Stock rotation should be defined by “first in, first out.”
According to American National Standards Institute (ANSI)/AAMI ST79, Comprehensive Guide to Steam Sterilization and Sterility Assurance in Healthcare Facilities, the criteria for a proper environment are:
• the room must be clean and made of solid materials that can withstand routine cleaning
• the ventilation should be under positive pressure with a minimum of 4 air exchanges per hour
• temperature should not be above 75°F, and humidity should not exceed 70%.
The 2015 AORN Guideline for Use of Packaging Systems for Sterilization states, “The shelf life of a packaged sterile item should be considered event-related. The sterility of an item does not change with the passage of time, but may be affected by particular events (eg, amount of handling) or environmental conditions (eg, humidity).”
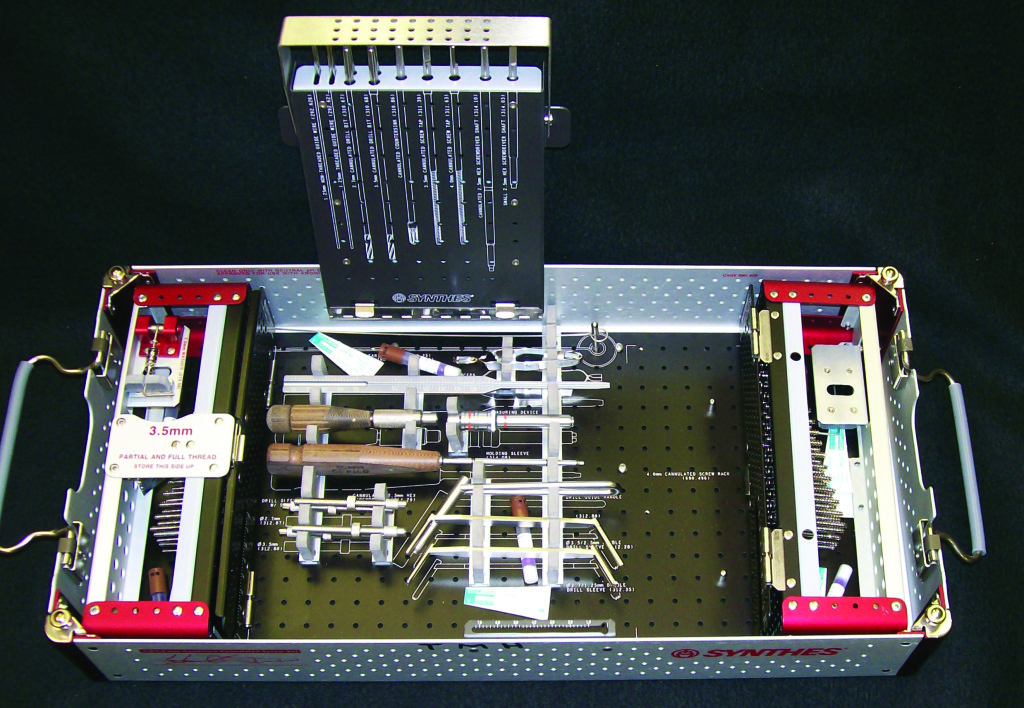
Product testing is done with chemical and biological monitors placed in areas that are most resistant to the sterilization process. Photo courtesy of Susan Klacik. Used with permission.
Instrument set weight, preparation, and verification
Sterilization is based on density. The total instrument set weight, including the weight of the instrument container, should not exceed 25 pounds. Because the sterilant must contact all surfaces, the instrument pan must be large enough to distribute all of the instruments so that they are exposed to the sterilant.
Dense instrument sets can cause wet packs from which the steam may not be able to escape the set. Wet packs are considered contaminated and cannot be used.
Chemical indictors are placed in the area of the instrument set that is least accessible to the sterilant—not necessarily in the middle or top (photo on p 27).
Product testing is used to ensure all areas of the instrument set—especially the areas most resistant to the sterilant—are properly sterilized. It should be performed when changing packaging methods, before placing new instrument sets into service, and when acquiring new loaner sets.
Chemical and biological monitors are placed in areas that are most resistant to the sterilization process with the location identified (photo at right). If using a sterilization container, contact the container manufacturer for the most resistant location. For loaner sets, the manufacturer may be able to provide this information.
Only the most challenging instrument set from a “loaner family” undergoes this test. The set should be labeled as a “test” so it is not sent for patient care. The package is sterilized according to the healthcare facility’s policy.
After sterilization, the physical parameters from the sterilizer are examined to ensure the sterilization parameters have been met. The package is opened, and quality monitors are examined. If any moisture is found, the cause must be identified and corrected.
The chemical indicators are examined to be sure they met their endpoint, and the biological monitors are incubated. All of these quality monitors must show a pass, thus verifying the set can be sterilized in the healthcare facility.

When wrapping with flat wraps, make sure the first fold completely covers the package all the way to the bottom of the instrument set, not just over the top of the set. Photo courtesy of Susan Klacik. Used with permission.
Flat wrappers
Flat wrappers come in different sizes so that items can be completely covered without leaving an excess of material that makes packaging cumbersome or causes steam to pool. Improper packaging can result in ineffective sterilant penetration into the package, wet packs, and inability to open the package aseptically.
The correct technique to use when wrapping with flat wraps is to make sure the first fold completely covers the package all the way to the bottom of the instrument set, not just over the top of the set (photo on p 29). The packaging should be snug to the instrument set. If the wrapper is too loose, it can create water puddles that result in wet packs or may leave openings that can cause contamination. If the package is too tight, it could hinder the sterilant from entering and leaving the package.
If there are any holes in the instrument set packaging, the packages are contaminated and cannot be used. The following practices can be used to avoid holes in packaging:
• Reduce the amount of instruments in a set. Heavy instrument sets are a common cause of packaging tears.
• Use the correct packaging weight. Heavy instrument sets require thicker packaging.
• Place corner protectors or tray liners between the instrument set and packaging to reduce holes that occur when instrument sets with sharp corners cut the wrapper.
• Place transport trays under the instrument set to facilitate handling after sterilization and avoid tears.
• Store heavy instrument sets on the easy to reach middle shelves rather than on very low or very high shelves. This provides for easy handling by the staff and prevents dragging the instrument set, which could cause tears.
• Store each instrument set on its own shelf, and do not stack instrument sets. Stacking can damage the wrapper material.
Sterilization containers
Not every container can sterilize everything, so it’s important to review the container manufacturer’s IFU, which will describe the types of instrumentation and material that can undergo sterilization.
Container manufacturers provide restrictions regarding lumen size and use of absorbent material. Sterilization containers should be loaded on the sterilizer cart below absorbent items to prevent absorbent items from getting wet from the container’s condensation.
Sterilization containers can be cleaned either mechanically or manually. They should be disassembled and cleaned before each use following the manufacturer’s IFU. The material used to construct a container can become damaged if the wrong chemicals such as bleach wipes are used for cleaning.
The container IFU will also provide instructions for quality checks and routine maintenance. One routine check is to inspect the container gasket with each use. If there are any breaches, such as cuts or staining, remove the gasket from service.
Paper-plastic peel pouches
Paper-plastic peel pouches are designed for packaging small, lightweight instruments. Not all paper-plastic peel pouches are validated for double pouching, so it is important to check with the manufacturer before double pouching.
If double pouching is permitted, pouches must be placed plastic to plastic for complete visibility, without any folds. If the internal chemical indicator is visible through the package, an external chemical indicator is not required.
Because the plastic side of the package is the stronger side, labeling is done there. Paper-plastic peel pouches should not be placed into an instrument set regardless of whether the set is wrapped or in a sterilization container. Placing paper-plastic peel pouches inside can obstruct air removal, steam contact, and drying. Steam cannot penetrate the plastic side of the pouch and thus prevents the sterilant from reaching all surfaces inside the package.
The sterilant enters and exits the package from the paper side. When the package is loaded onto the sterilizer cart, it must be loaded on its side to permit the sterilant to both enter and exit. As with all types of packages, these too must be loaded loosely so that the sterilant can circulate. Paper-plastic peel pouches can be loaded into baskets to hold them up. There are also holders designed specifically to sterilize these packages on their side.
Wrapping it up
Sterilization packaging is an important part of the sterilization process. During the packaging selection step, the user should consult the packaging manufacturer’s IFU to ensure compatibility with the sterilization method.
Maintaining the integrity of the sterile package requires following best practices as described in the ANSI/AAMI ST79 and AORN guidelines listed in the references below. ✥
 Susan Klacik, BS, CRCST, CIS, ACE, FCS, is president, Klacik Consulting, in Canfield, Ohio, and CSS manager at St Elizabeth Health Center and the IAHCSMM (International Association of Healthcare Central Service Materiel Management) representative to AAMI (Association for the Advancement of Medical Instrumentation).
Susan Klacik, BS, CRCST, CIS, ACE, FCS, is president, Klacik Consulting, in Canfield, Ohio, and CSS manager at St Elizabeth Health Center and the IAHCSMM (International Association of Healthcare Central Service Materiel Management) representative to AAMI (Association for the Advancement of Medical Instrumentation).
References
AORN. Guidelines for perioperative practice, guideline for selection and use of packaging systems for sterilization. Denver, CO: AORN, 2015.
Association for the Advancement of Medical Instrumentation. Comprehensive guide to steam sterilization and sterility assurance in health care facilities. ANSI/AAMI ST79: 2010 & A1: 2010 & A2: 2011 & A3: 2012 & A4: 2013. www.aami.org.
Premarket notification [510(k)] submissions for medical sterilization packaging systems in health care facilities; Draft guidance for industry and FDA draft released for comment on March 7, 2002.

 Free Daily News
Free Daily News