Editor's Note The Food and Drug Administration (FDA) on September 13 announced the recall by OriGen Biomedical (Austin, Texas) of two lots of its VV28F Reinforced Dual Lumen ECMO [extracorporeal membrane oxygenation] Catheters (Catalog number VV28F, Lots N18487 and V18487-1). The recall was initiated because the catheters have the potential…
Editor's Note Infection control practices that focus on perioperative patient skin and wound hygiene and transparent display of surgical site infection (SSI) data, not OR attire policies, were associated with lower SSI rates in this multi-center study. A total of 20 American College of Surgeons National Surgical Quality Improvement Program…

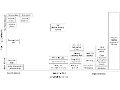
Editor's Note The Joint Commission on September 13 announced a new Sentinel Event Alert that focuses on inadequate hand-offs and tips to improve them. A common problem with hand-offs is communication, which is the focus of “Sentinel Event Alert, Issue 58: Inadequate hand-off communication.” The alert includes an infographic of…

Editor's Note From 2008 to 2014, rates of postoperative readmissions declined for both Hospital Readmission Reduction Program targeted procedures (total hip and total knee replacements)--from 6.8% to 4.8%--and nontargeted procedures (colectomy, lung resection, abdominal aortic aneurysm repair, coronary artery bypass graft, aortic valve replacement, and mitral valve repair)--from 17.1% to…
Editor's Note Officials at Children’s Hospital Colorado in Aurora are sending notifications to nearly 3,400 patient families about a data breach that may have compromised their health information, according to the September 11 Becker’s Health IT & CIO Review. On July 11, investigators found an unauthorized person may have accessed…

Editor's Note The Food and Drug Administration (FDA) is filling 13 positions in a new digital health unit that was created as part of the FDA Reauthorization Act of 2017, the September 11 FierceHealthcare reports. The FDA intends to build a group of experts who are experienced with software lifecycle…

Editor's Note The transoral endoscopic thyroidectomy vestibular approach can be safe for select patients, with outcomes similar to those of the open approach, this study finds. Of 425 patients analyzed, the operative time for the transoral approach was longer than the open approach (100.8 vs 79.4 minutes) but transoral patients…
Editor's Note The Food and Drug Administration (FDA) on September 6 categorized the recall by Datascope/MAQUET of its CS100i, CS100, and CS300 intra-aortic balloon pumps as Class I, the most serious. The recall was initiated because of a false blood detection alarm and ingress of fluid into the balloon pump.…

Editor's Note In this study, preadmission clinical frailty independently predicted adverse discharge destination (ie, death or discharge to a long-term, chronic or acute care facility) in geriatric trauma patients. The analysis of 266 trauma patients 65 years and older using the Clinical Frailty Scale (CFS) and the laboratory Frailty Index…
Editor's Note The Food and Drug Administration (FDA) on September 7 announced the recall by Genetech (South San Francisco, California) of three lots of Activase (alteplase) 100 mg vials that were co-packaged with sterile water for injection. The vials of water, manufactured by Hospira Inc (Lake Forest, Illinois), may be…