
Editor's Note A personalized digital tool is helping patients and orthopedic surgeons make more informed, confident decisions about total knee replacement (TKR), according to BMC Health Services Research October 21. Both patients and surgeons reported the EKIT tool—a tablet-based decision aid developed in Germany—improves shared decision-making (SDM), enhances communication, and…
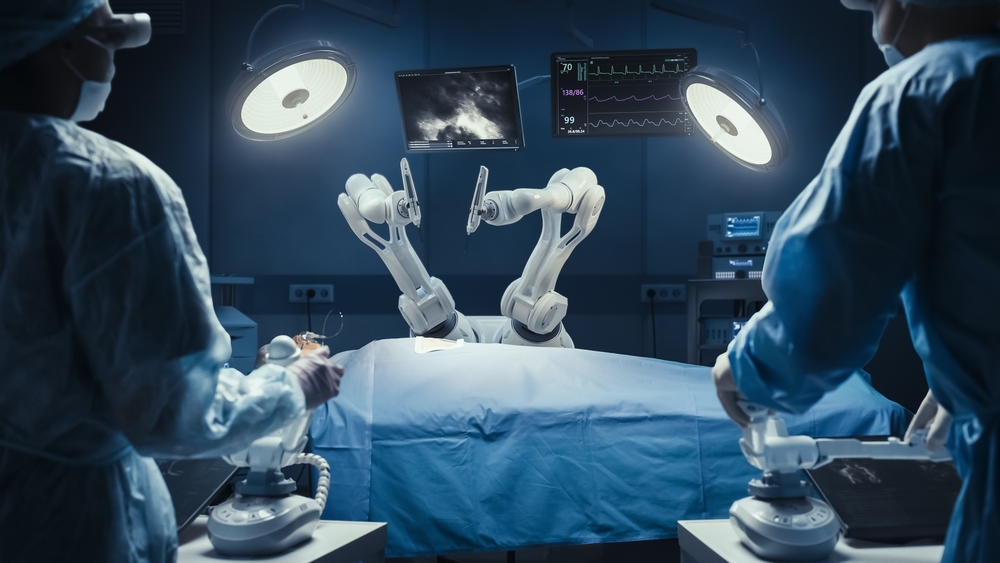
Robotic surgery has moved from cutting-edge to commonplace. The question is no longer whether to use robotics but when to introduce it and how to ensure adoption is efficient, affordable, and seamless for surgical teams. Ambulatory surgery centers (ASCs) are increasingly adding robotics to their service lines, driven by the…

Editor's Note The Food and Drug Administration (FDA) on October 10 classified a cybersecurity correction involving Abiomed’s Automated Impella Controller as a Class I recall, the most serious type, according to the FDA Medical Device Recalls and Early Alerts database. While devices are not being removed from clinical settings, the…

The centralization of medical device processing to one facility is becoming more prevalent. Centralizing sterile processing activities reduces expenses while concentrating expertise. However, this also introduces new concerns. When sterile processing is located within the same building where instrumentation is used, transport occurs over smooth floors in a controlled environment…

Editor's Note The US Department of Commerce has initiated national security investigations that could trigger new tariffs on a wide range of imported medical products, with potentially far-reaching effects for healthcare providers, Reuters September 24 reports. The probes, opened under Section 232 of the Trade Expansion Act of 1962, cover…

Editor's Note Surgical quality leaders are pushing boundaries with new strategies that blend technology, frontline engagement, and national-local collaboration. That was the message from the American College of Surgeons (ACS) Quality and Safety Conference (QSC), held July 17–20, 2025, in San Diego, according to a September 10 ACS report. The…

Editor's Note Innovation is transforming surgical care faster than most institutions can keep pace, but leaders must distinguish between investments that advance patient care and those that add cost without meaningful benefit. That is the central message from a September 8 Harvard Medical School article featuring insights from Jon O.…

Editor's Note Colorectal tumors once considered inoperable are now routinely treated with curative surgery, thanks to advances in multimodality therapy and complex resection techniques, Mayo Clinic September 16 reports. Decades ago, cancers invading the sacrum, pelvic organs, or major blood vessels were often deemed unresectable, leaving patients with only palliative…

Editor's Note The American Medical Association (AMA) has released the 2026 Current Procedural Terminology (CPT) code set, introducing 288 new codes that capture emerging technologies and modern care delivery models, the association announced on September 11. In total, the update includes 418 changes: 84 deletions, 46 revisions, and the newly…

Editor's Note Hospitalized surgical patients in 2024 were nearly 20% more likely to survive than expected compared to 2019, according to an August 5 analysis from the American Hospital Association (AHA) and Vizient. The report credits safety improvements such as reductions in infections, falls, and major complications, even as surgical…