
Editor's Note New and revised sterilization and reprocessing standards are reshaping the landscape for sterile processing departments, placing greater emphasis on chemical modalities, device-specific protocols, and system-wide quality management, according to the Healthcare Purchasing News May 27 update on compliance and standards. Among the most significant developments is the overhaul…
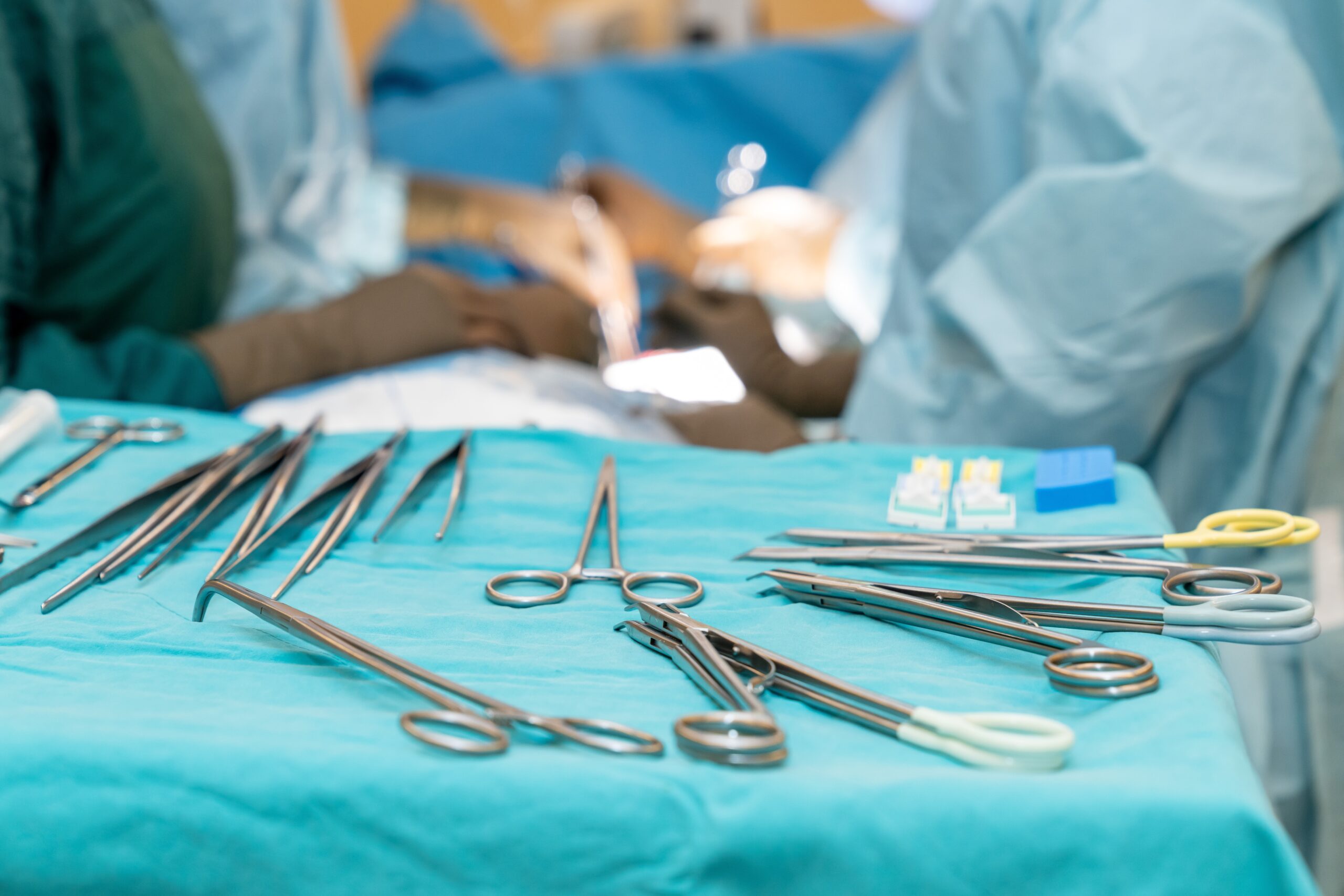
Preventing infection from contaminated surgical tools requires attention to every link in a complex chain of processes, from point-of-use pretreatment in the OR to the moment the freshly disinfected or sterilized item arrives at the next patient’s bedside. For those on the front lines, manufacturers’ written instructions for use (IFUs)…

Editor's Note Air quality in cardiac ORs may be a silent driver of surgical site infections (SSIs), with airborne contamination linked to significantly elevated infection risk and mortality—especially when ventilation is suboptimal. A newly published study covered by Medical Dialogues May 19 reveals that one-third of bacteria in cardiac procedures…

Editor's Note Unnecessary traffic, workflow interruptions, and lapses in protocol in the OR increase the risk of surgical site infections (SSIs), according to an April 23 article in Infection Control Today. While sterile technique, antibiotic use, and instrument cleanliness remain front-line defenses against infection, authors Katharine J. Hoffman, MPH, CIC, and…

Editor's Note The US Food and Drug Administration has designated DeRoyal Industries’ recall of GeoMed custom tracecarts a class 1, the most serious type of recall indicating a risk of serious injury or death. According to the April 24 FDA notice, the recall is due to sterility concerns with the…
The US Food and Drug Administration receives more than 100,000 medication-related reports, and some of them, according to the AORN Journal, “involve patient death.” AORN’s 2024 updated guidelines include safety updates for perioperative staff when handling, transporting, and administering medication, which can be a complex process prone to errors. In…