
Editor's Note The Trump administration paused new NIH research grants and contracts, prompting widespread concern before abruptly reversing course, according to a July 30 article in Forbes. Citing a separate report in STAT, the article details how the Office of Management and Budget (OMB) directed the NIH to suspend new…

Editor's Note A recent article from HIT Consultant highlights findings from Incredible Health’s 2025 State of US Nursing & Technicians Report, revealing mounting strain across the nursing and healthcare technician workforce. Reportedly based on insights from more than 1 million professionals, findings include: 71% of nurses report that staffing shortages…

Editor's Note A July 16 study published in BMC Research Notes found that mental fatigue among perioperative nurses is significantly associated with increased rates of missed perioperative nursing care. This cross-sectional study surveyed 385 operating room nurses working in university-affiliated hospitals in East Azerbaijan, Iran. Participants met inclusion criteria related…

Editor's Note A federal judge has ordered the Trump administration to restore health-related webpages and datasets removed under a January executive order, according to a July 29 article in Medscape. The ruling follows a lawsuit by Doctors for America and the city and county of San Francisco, which argued that…

Editor's Note The Joint Commission has launched a new strategy to revise its accreditation and certification programs to better reflect the distinct needs of pediatric care. According to a July 29 announcement, the effort responds to requests from the children’s health community and aims to revise or remove standards that…
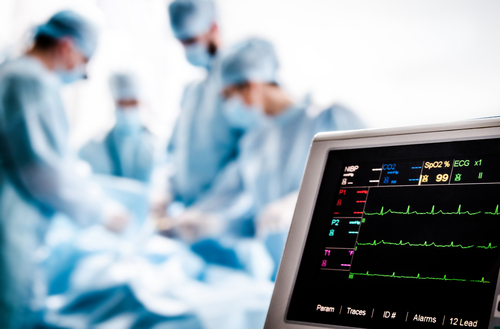
While most emergency surgical procedures are carried out uneventfully and safely, the OR is also a place where potentially life-threatening and least-expected instances can arise. Emergencies such as malignant hyperthermia, intraoperative cardiac arrest, and anaphylaxis can catch OR leaders and staff off guard. Perioperative teams need proper and adequate preparation…

Editor's Note US News & World Report has named 504 hospitals across 49 states and 95 metro areas to its 2025–2026 list of Best Regional Hospitals, recognizing facilities with superior performance in patient outcomes, nursing care, and procedural quality. The announcement, made July 29, marks the 36th edition of the…

Editor's Note Postoperative delirium significantly worsens outcomes for older adults undergoing major noncardiac surgery, according to research published July 8 in JAMA Network Open. Specifically, findings showed patients who developed postoperative delirium had 3.5 times the odds of death or major complications, 2.8 times the odds of 30-day mortality, and…

Editor's Note Glucagon-like peptide-1 (GLP-1) receptor agonists may offer orthopedic patients with obesity and type 2 diabetes a powerful tool for preoperative weight loss and potential disease modification, according to a July 10 review article in The Journal of Bone and Joint Surgery. However, the agents carry perioperative risks that…

Editor's Note The US Food and Drug Administration (FDA) has designated the recall of Ethicon Endo-Surgery, LLC’s Endopath Echelon Vascular White Reload for Advanced Placement Tip a Class I, the most severe category indicating risk of serious injury or death. As detailed in the agency’s July 25 announcement, the recall…