Healthcare industry groups criticize federal cybersecurity reporting rule

Editor's Note Healthcare industry groups are calling for the federal government to streamline and ease the recently proposed cybersecurity incident reporting rule by the Cybersecurity and Infrastructure Security Agency (CISA), Fierce Healthcare reported July 8. According to the report, CISA's proposal imposes enhanced reporting requirements for critical infrastructure entities, including…
Justice Department announces nationwide action on healthcare fraud enforcement

Editor's Note On June 27, The Justice Department announced the 2024 National Health Care Fraud Enforcement Action, leading to criminal charges against 193 defendants, including 76 medical professionals across 32 federal districts in the U.S. for their involvement in various health care fraud schemes. These schemes accounted for approximately $2.75…
New requirements for ASCs on life safety, patient rights effective August

Editor's Note Starting August 1, ambulatory surgical centers (ASCs) will need to comply with new and revised requirements for Life Safety Code® and patient rights, a June 26 news update from The Joint Commission reports. These updates align with the Centers for Medicare & Medicaid Services (CMS) Conditions for Coverage.…
Supreme Court decision could lead to legal challenges to payments, risks to healthcare regulation

Editor's Note Food and Drug Administration (FDA) regulations are at risk, and legal challenges to Medicare payments are likely to rise following the Supreme Court’s overturning of the 40-year-old legal precedent Chevron deference, according to a June 28 report in Becker’s Hospital Review. "Chevron deference is the principle that when…
Study: Federal antitrust action minimal relative to number of hospital mergers

Editor's Note Federal regulation of hospital mergers is inadequate, according to an April antitrust enforcement study scheduled to be published by the American Economic Association. According to a June 14 report in Modern Healthcare, researchers at universities including Harvard and Yale analyzed insurance claims data from Aetna, Humana, and UnitedHealthcare,…
Scaling standards from sterile processing department to clinic
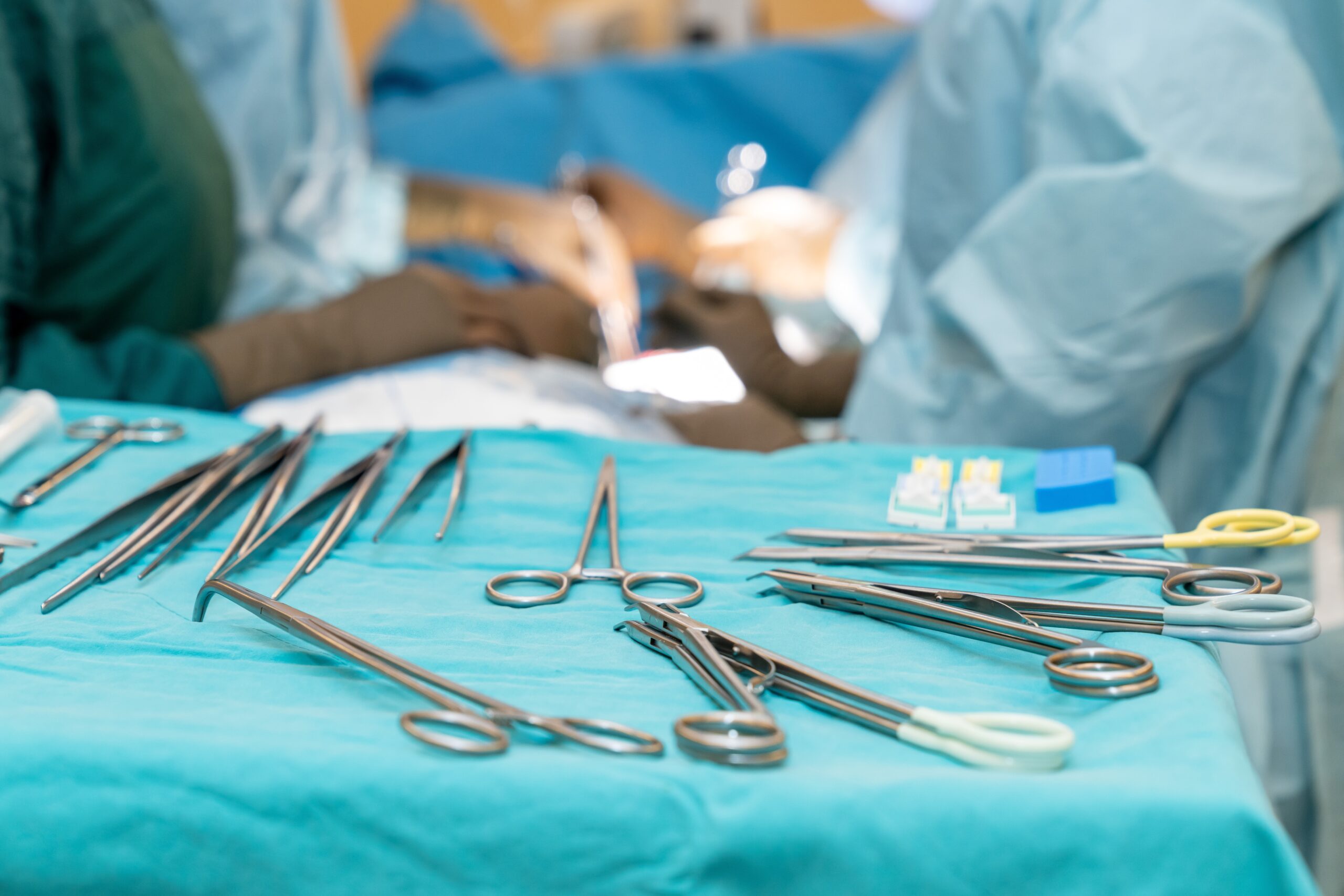
Reforming instrument reprocessing practices does not always end with the main sterile processing department (SPD). Holding clinics to the same standard adds to the challenge, whether they are associated with hospitals or operate independently. Nonetheless, standardization is just as essential to maintaining efficiency and quality standards. Whether a clinic is…
Updated ethylene oxide sterilizer standard covers latest technology

Editor's Note The Association for the Advancement of Medical Instrumentation (AAMI) has updated its standard on ethylene oxide (EO) sterilizers for healthcare facilities, according to a June 12 press release. The first update in two decades, the fourth edition ANSI/AAMI ST24:2024 covers labeling, safety, performance, and testing requirements for general-purpose…
Announcing the ASC administrators CASC training course

Editor's Note The OR Manager Conference is proud to announce it will offer a 1-day training course outlining core areas of study for the Certified Administrator Surgery Center (CASC) exam to earn the CASC credential. This training is ideal for those responsible for the operations of running an ambulatory surgery…
Phased-in nurse staffing ratio law draws complaints in Oregon

Editor's Note The state agency overseeing Oregon’s hospitals has received a “flood of complaints” due to a “first-of-its-kind” law mandating progressively stricter nurse and certified nursing assistant (CNA) staffing ratios, according to a June 7 report in KMTR. Passed after extensive negotiations among hospital executives, staff, and nurse unions…
Hospital CEOs lend expertise to White House gun violence meeting

Editor's Note A June 6 meeting on gun violence prevention at the White House attracted more than 80 top health care executives to lend expertise on mental health, gunshot wounds, and more, Becker’s Hospital Review reports. Hosted by the White House Office of Gun Violence Prevention, established in 2023,…

 Free Daily News
Free Daily News