
Editor's Note Recent recalls of the Ballard Closed Suction Systems from Avanos Medical Inc., Infant Heated Wire Circuits from AirLife/Vyaire, and Broselow Pediatric Emergency Rainbow Tape from AirLife have been designated as Class 1, the US Food and Drug Administration’s (FDA’s) most severe category indicating risk of serious injury or…

Editor's Note Recent early alerts from the US Food and Drug Administration (FDA), issued when the agency becomes aware of potentially high-risk issues, involve Abiomed’s Automated Impella Controller (AIC) and infusion pump software from Baxter. The AIC system, which is the user control interface for the Impella catheter blood pump,…

Editor's Note The US Food and Drug Administration (FDA) has designated Cook’s recent recall of the Beacon Tip 5.0 Fr Angiographic Catheter as a Class 1, the most severe category indicating serious risk of injury or death. The recall was reportedly motivated by reports of tip separation both prior to…

Editor's Note The US Food and Drug Administration (FDA) has designated recent medical device recalls involving GE Healthcare’s Carestation anesthesia system, Medtronic aortic root cannula systems, Zoll Circulation’s AutoPulse NXT Resuscitation System, and Medtronic’s Bravo CF Capsule Delivery Devices as Class 1, the most severe category indicating serious risk of…

Editor's Note The US Food and Drug Administration (FDA) has issued three Class I medical device recalls—the most severe category indicating risk of serious injury or death—for Q’Apel Medical Inc.’s HIPPO 072 Aspiration System and Cheetah catheter, Fresenius Kabi USA’s Blood Products Administration Set with 200 Micron Filter, and Medline…

Editor's Note New and revised sterilization and reprocessing standards are reshaping the landscape for sterile processing departments, placing greater emphasis on chemical modalities, device-specific protocols, and system-wide quality management, according to the Healthcare Purchasing News May 27 update on compliance and standards. Among the most significant developments is the overhaul…

Editor's Note Centerline Biomedical’s recall of IOPS Guidewire ATW-2, Zyno Medical’s recall of Z-800 series infusion pumps, and Nipro’s recall of MedicaLyte Liquid Bycarbonate Concentrate have been designated by the US Food and Drug Administration (FDA) as Class 1, the most serious designation indicating risk of severe injury or death.…

Editor's Note Baxter has issued a correction notice for its Novum IQ Large Volume Pump (LVP) after identifying a serious risk of underinfusion linked to the device’s standby mode and power-off conditions. First published on April 24 on the Food and Drug Administration (FDA) website and subsequently reported by Healthcare…
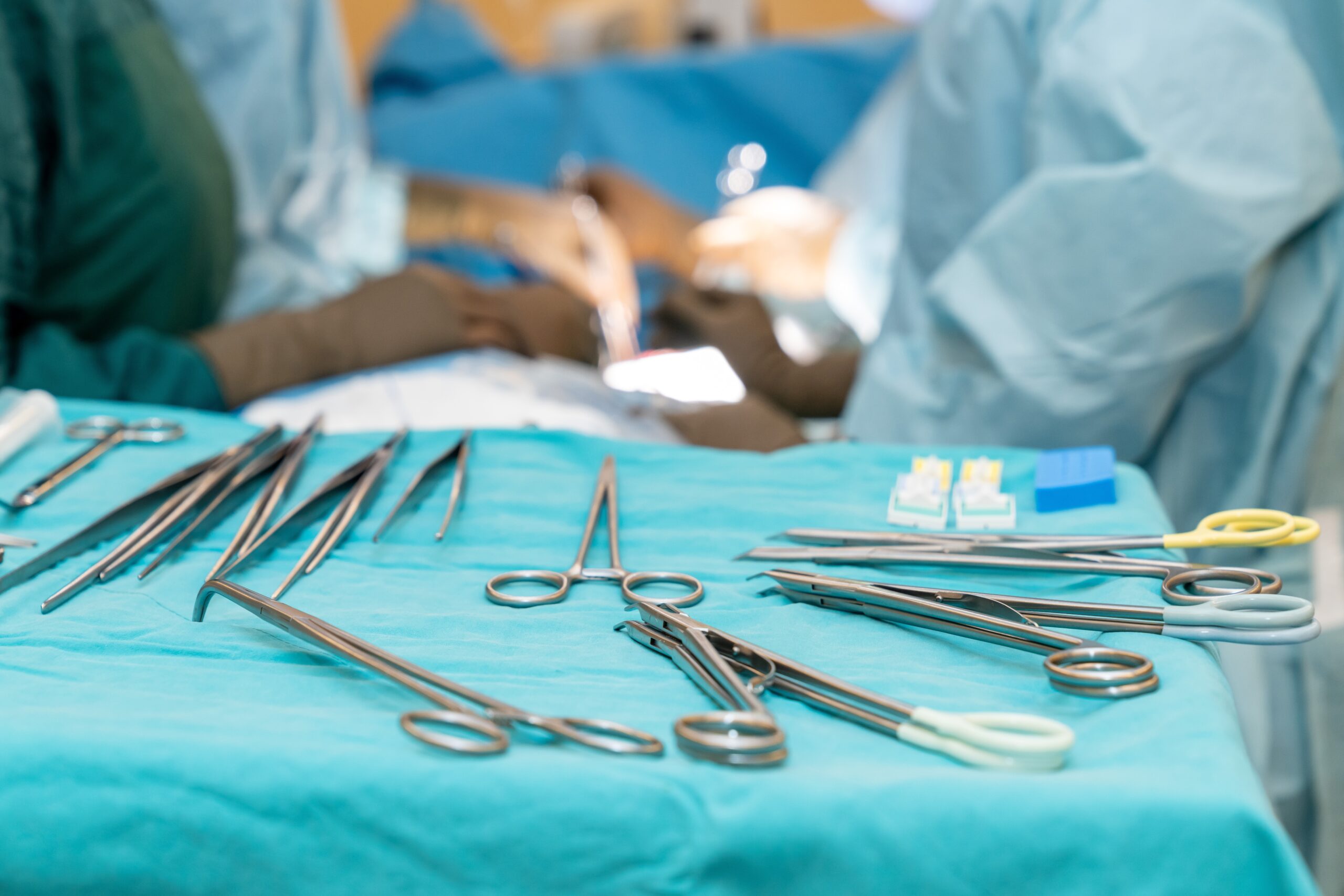
Preventing infection from contaminated surgical tools requires attention to every link in a complex chain of processes, from point-of-use pretreatment in the OR to the moment the freshly disinfected or sterilized item arrives at the next patient’s bedside. For those on the front lines, manufacturers’ written instructions for use (IFUs)…

Editor's Note Surgeons across top US health systems are swapping traditional monitors and even robotic systems for augmented reality (AR) headsets, which are streamlining procedures, enhancing precision, and slashing costs, Modern Healthcare May 27 reports. As detailed in the article, AR headsets are rapidly emerging as valuable surgical tools. From…