APIC calls for improved medical device reprocessing instructions, guidelines

Editor’s Note The Association for Professionals in Infection Control and Epidemiology (APIC) is advocating for clearer reprocessing instructions for medical devices to improve patient safety and efficiency, Outpatient Surgery Magazine August 19 reports. Many current instructions for use (IFUs) are considered overly complex, outdated, and difficult to interpret, especially for…
Scaling standards from sterile processing department to clinic
Reforming instrument reprocessing practices does not always end with the main sterile processing department (SPD). Holding clinics to the same standard adds to the challenge, whether they are associated with hospitals or operate independently. Nonetheless, standardization is just as essential to maintaining efficiency and quality standards. Whether a clinic is…
Ambulatory endoscopy management strategies keep patients, finances healthy
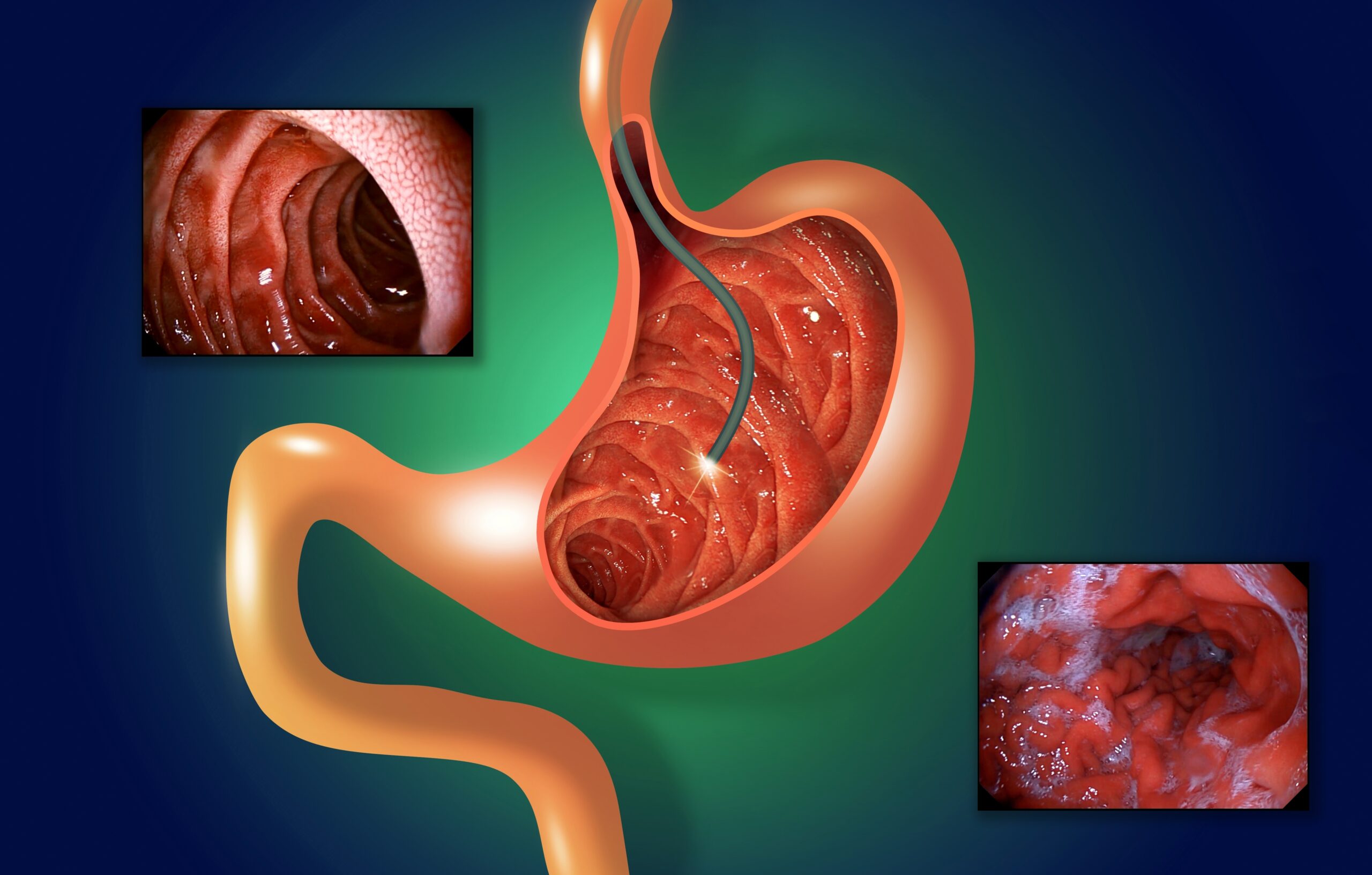
Gastrointestinal (GI) endoscopy is one of the most common procedures in the US. Performed more than 17.1 million times per year in inpatient and outpatient hospital settings as well as ambulatory surgery centers (ASCs), GI procedures account for 68% of all endoscopies, according to a May 2022 article in Digestive…
Common inspection points for surgical instrumentation
Inspecting surgical devices is a time-consuming process. However, diligently checking every instrument prior to sterilization is essential to ensuring safe, proper functioning. As the last people to see devices before they are used for patient care, sterile processing technicians must be thorough. Exterior surfaces should be inspected for flaws such…
Managing immediate use steam sterilization

Immediate use steam sterilization (IUSS, a standard steam sterilization cycle with little to no dry time) is considered safe for patient care when the processes recommended in Association for the Advancement of Medical Instrumentation (AAMI) standards and AORN guidelines are followed. IUSS is a valuable option in an emergency. Lack…
Utility of lighted magnification, borescopes to inspect flexible endoscopes
Editor's Note This study led by epidemiologist Cori L. Ofstead, MSPH, and associates, St Paul, Minnesota, found visible damage and residue or debris in 100% of 25 processed flexible endoscopes, using a new visual inspection program that included magnification and borescopes. Fully processed endoscopes were examined twice during a 2-month…
New AMDR report strengthens case for single-use medical device reprocessing

Recent analysis provided by the Association of Medical Device Reprocessors (AMDR) indicates that US hospitals could save up to $2.28 billion a year by maximizing the use of reprocessed single-use medical devices. According to the report, in 2020, US hospitals saved $372 million just by reprocessing single-use medical devices, as…
Tackling internal objections to single-use device reprocessing
Many healthcare professionals are aware of single-use device reprocessing and its benefits. According to the Association of Medical Device Reprocessors, the savings generated by such programs can strengthen a facility’s financial sustainability and help provide a path for more responsible environmental stewardship. Sometimes, ambulatory surgery centers (ASCs) may experience situations…

 Free Daily News
Free Daily News