New research shows health risks of smoke exposure in gastrointestinal endoscopy

Editor's Note Research presented at this year’s Digestive Disease Week in May highlights the potential health risks posed by smoke generated during tissue-cutting ablations in gastrointestinal (GI) endoscopy, Gastroenterology & Endoscopy News May 18 reports. Unlike surgeons in ORs, who follow specific regulations to mitigate smoke exposure, GI endoscopy procedures…
Post-CABG cardiac shockwave therapy shows promise in early study

Editor's Note Using a device they call a “space hairdryer,” researchers in Austria applied gentle shockwaves to regenerate heart tissue after coronary artery bypass graft surgery (CABG) in a study with potential implications for millions of patients, BBC News reported June 20. Researchers are now seeking larger trials, European regulatory…
FDA announces Class 1 recalls for patient return electrodes, intra-aortic balloon catheters, anesthesia systems

Editor's Note The US Food & Drug Administration (FDA) has announced Class 1 recalls—the most severe category, indicating risk of serious injury or death—for three products: MEGA SOFT Pediatric Patient Return Electrodes from Megadyne, Vaporizer Sevoflurane Maquet Filling from Getinge; and Arrow FiberOptix Intra-Aortic Balloon Catheter Kit and Arrow UltraFlex…
Common inspection points for surgical instrumentation
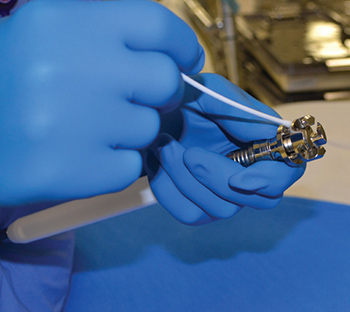
Inspecting surgical devices is a time-consuming process. However, diligently checking every instrument prior to sterilization is essential to ensuring safe, proper functioning. As the last people to see devices before they are used for patient care, sterile processing technicians must be thorough. Exterior surfaces should be inspected for flaws such…
Unveiling ECRI’s 2024 top 10 health technology hazards list

What is the purpose of the top 10 health technology hazards list, released every year by ECRI? “Our number one goal at ECRI is to reduce preventable harm,” stresses Jason Launders, MSC, former director of operations, device evaluation, at ECRI. “We know that every healthcare provider has a lot they…
Burns prompt recalls of airway scopes, electrosurgical pads
Editor's Note Reports of burn injuries have prompted two medical device recalls: one for Megadyne patient return electrodes and one for Olympus bronchofiberscopes and bronchovideoscopes. According to a December 21 notice from AORN, the former recall is a voluntary correction on the part of Megadyne Medical Products, Inc. Following reports…
New Sentinel Event Alert focuses on preventing surgical fires
Editor's Note A new Sentinel Event Alert from The Joint Commission focuses on the continuing dangers of surgical fires, why they occur, and how to take preventative measures. The alert was issued on October 18. Some highlights include: There is no national repository collecting data on surgical fires, and little…
FDA: Class I recall of Megadyne’s Mega 2000, Mega Soft reusable patient return electrodes
Editor's Note The Food and Drug Administration (FDA), on July 11, identified the recall by Megadyne of its Mega 2000 and Mega Soft reusable patient return electrodes as Class I, the most serious. The recall was initiated after Megadyne received reports of pediatric and adult patients receiving burn injuries during…
Other requirements for processing electrosurgical instruments

The use of electrosurgical instrumentation is common in minimally invasive surgery (MIS). However, while these instruments are common, processing them is complex and time-consuming. Insulated electrosurgical instrumentation can cause patient harm, including an electrical burn, if there is a breach in the insulation. These instruments are designed to have an…

 Free Daily News
Free Daily News