Single-use items contribute two-thirds of carbon footprint in the OR
Editor's Note In this study from the UK, researchers find that two-thirds of carbon contributions in the OR can be attributed to single-use items and one-third to reusable products used in five common surgical procedures. The mean average carbon footprint includes: 85.5 kg CO2e (carbon dioxide equivalents) for knee arthroplasty…
New study: Disposable gowns may expose clinicians to infection
Editor's Note After this October 2020 peer-reviewed study, titled “Disposable versus reusable medical gowns: A performance comparison,” found that “isolation gowns commonly worn in medical units or intensive care units ripped too easily and allowed about four to 14 times the expected amount of liquid to seep through when sprayed…
Creative recycling: blue wrap, bottle caps, thinking outside the box
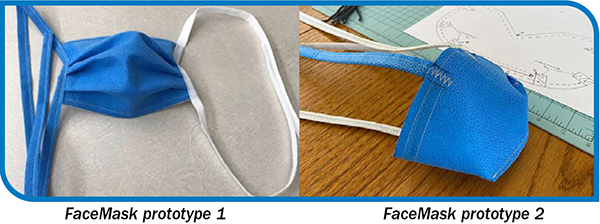
There is more than one way to skin a blue wrap—and transform its utility. Faced with overwhelming amounts of waste, teams in operating rooms are finding creative solutions, taking discarded medical supplies and repurposing them into bed pans, COVID masks, or in some cases, works of art. For many health…
ACS updates efforts to help Ukraine
Editor's Note In a March 28 update, the American College of Surgeons (ACS) says it is following the humanitarian crisis unfolding in Ukraine through Operation Giving Back, and that no current role or mechanism exists for safe travel to help in person at this time. ACS notes that healthcare facilities…
NIOSH rescinds approval for Pacific PPE N95 respirator masks
Editor's Note The National Institute for Occupational Safety and Health (NIOSH) announced on March 28 that it had honored a request by Pacific PPE Corp to rescind all of its N95 respirator mask approvals, effective immediately. Respirators with NIOSH approval numbers TC-84A-9278, TC-84A-9299, and TC-84A-9313 will no longer be manufactured,…
The Joint Commission issues Quick Safety on instrument reprocessing
Editor's Note The Joint Commission on February 14 issued a new Quick Safety on “Ensuring critical instruments and devises are appropriate for reuse.” The Quick Safety highlights reprocessing guidance from the Food and Drug Administration (FDA) as well as special considerations for single use devices reprocessed by third parties that…
WHO: Dire need to deal with huge volumes of COVID-19 medical waste
Editor's Note Tons of extra medical waste generated in response to COVID-19 has put tremendous strain on healthcare waste management systems, threatening human and environmental health, according to a February 1 World Health Organization (WHO) report. In this global analysis WHO says: A large share of 87,000 tons of personal…
FDA revokes EUA for non-NIOSH-approved respirators
Editor's Note The Food and Drug Administration (FDA) on June 30 announced that it is revoking the Emergency Use Authorizations (EUAs) for non-NIOSH-approved disposable respirators (including N95s), effective July 6. The announcement also revokes EUAs for decontamination and bioburden reduction systems for disposable respirators, effective immediately. The FDA says the…
Researchers raise the alarm on splashing during reprocessing--Part 1
Does it really matter if surgical instruments are submerged in cleaning solution when technologists or nurses scrub them after a case? Is there a reason for the 3-foot separation between dirty and clean areas? Do germs stop at the red line? During the COVID-19 pandemic, much attention has been focused…
FDA clears single-use flexible cystoscope from UroViu
Editor's Note UroViu Corporation, Bellevue, Washington, announced May 24 that it has received clearance from the Food and Drug Administration (FDA) for its portable, flexible single-use cystoscope. The Uro-G has a fully deflectable tip that enables physicians to perform interventional and diagnostic urologic procedures in various settings, without reprocessing.

 Free Daily News
Free Daily News