 Supply Chain/Technology
Supply Chain/Technology
FDA: Class I recall of Atrium Medical’s iCast Covered Stent
Editor's Note The Food and Drug Administration (FDA) on June 2 identified the recall by Atrium Medical Corporation of its iCast Covered Stent System as Class I, the most serious. The recall was initiated because of increased complaints about the separation of the balloon or catheter hub from the delivery…
FDA: Class I recall of Arjo Sara Plus lift
Editor's Note The Food and Drug Administration (FDA) on May 27 identified the recall of the ArjoHuntleigh Polska Sara Plus floor lift as Class I, the most serious. The recall was initiated because of the risk of smoke or fire if the lift is used when the battery is low.…
FDA: Class I recall of Abbott’s Dragonfly OpStar Imaging Catheter
Editor's Note The Food and Drug Administration (FDA) on May 26 identified the recall of Abbott Medical’s Dragonfly OpStar Imaging Catheter as Class I, the most serious. Abbott is recalling certain lots of the imaging catheter because the marker band farthest from the catheter tip (proximal marker) may become loose…
ECRI: 2022 winners of Alerts Impact Award
Editor's Note ECRI on May 25 announced the three winners and three finalists of its 2022 Alerts Impact Award for excellence in recall management. The annual award is given to those who have demonstrated strong success in implementing recall management programs in their healthcare organizations. The winners are: Eskenazi Health,…
Private sector, federal agency experts join Biden Administration to boost healthcare supply chain
Editor's Note The US Department of Health and Human Services (HHS) has engaged experts from the private sector and various government agencies to “share information and best practices, identify threats, and mitigate risks” regarding the US supply chains, including for healthcare, according to an Office of the Assistant Secretary for…
Five things to ask distributors about ASCs
While the COVID-19 pandemic continues to evolve with impacts shifting over time, some of the changes it brought to healthcare—specifically the OR—may be permanent. One trend it accelerated: the rise in new ambulatory surgery centers (ASCs), which specialize in elective or same-day outpatient surgical procedures. While this trend was largely…
Session: Right-size your surgical supply inventory
Editor's Note In this session, Brooke Mullett, MBA, senior director of operations for perioperative and surgical services at Cincinnati Children’s Hospital Medical Center (CCHMC), and Ash Crowe, project manager at St. Onge Company, walked attendees through a $100 million renovation project of the CCHMC ORs and surgical supply inventory. Even…
ECRI announces 2022 Health Technology Excellence Award
Editor's Note On May 5, McLaren Northern Michigan was named the winner of ECRI’s 16th Health Technology Excellence Award for its integration of patient-care technologies into new hospital construction. The award-winning submission described the hospital’s plan to build 200 private patient rooms, a range of clinical care areas, as well…
Hospitals, ORs fear ransomware fallout from Ukraine invasion
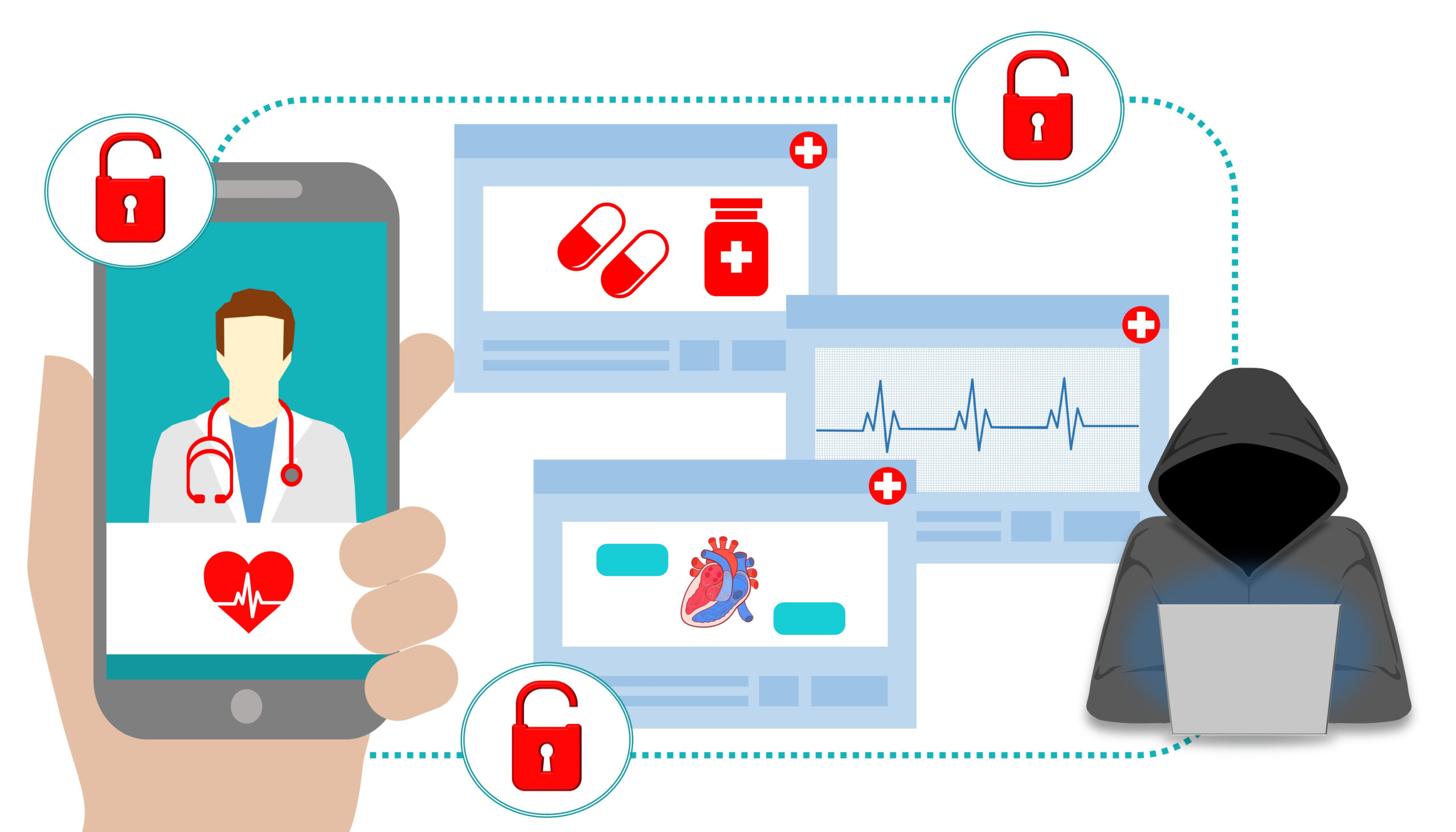
Hospitals and health systems know they are attractive targets for cybercriminals. When lives are at stake, and the victims are often insured, ransomware gangs can expect a quick and easy payout. But since the Russian invasion of Ukraine on February 24, hospitals have had to face a new reality: The…
Creative recycling: blue wrap, bottle caps, thinking outside the box
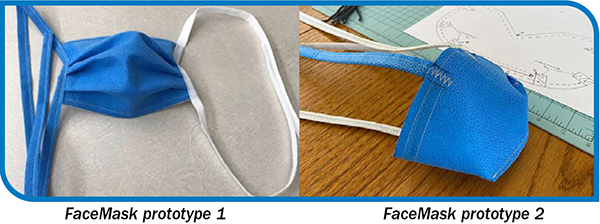
There is more than one way to skin a blue wrap—and transform its utility. Faced with overwhelming amounts of waste, teams in operating rooms are finding creative solutions, taking discarded medical supplies and repurposing them into bed pans, COVID masks, or in some cases, works of art. For many health…

 Free Daily News
Free Daily News