 Supply Chain/Technology
Supply Chain/Technology
FDA adds AEDs, chest drains to device shortage list
Editor's Note The Food and Drug Administration (FDA) on July 19 added automated external defibrillators (AEDs) and chest drains/suction canisters and autotransfusion systems to its list of medical device shortages during COVID-19. The shortage of chest drains/suction canisters and autotransfusion systems is because of increased demand. The shortage of AEDs…
A team approach to reducing instrumentation, costs during the age of COVID-19
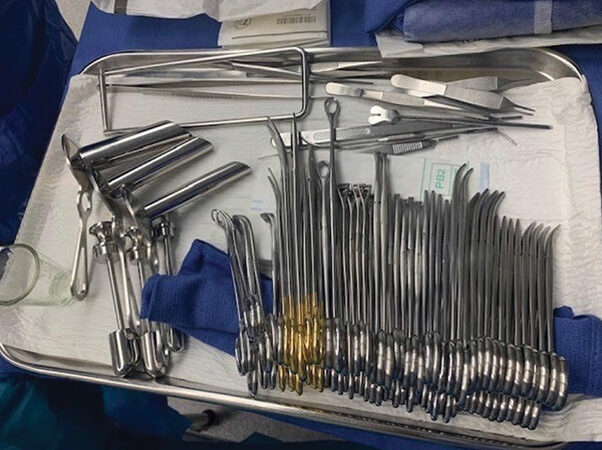
ORs rebounding from the financial impact of COVID-19 are looking for ways to reduce expenses. With rising costs coming from all angles of the economy, some have adopted systemwide processes to reduce, streamline, and improve surgical instrumentation sets. Two major health systems, the Cleveland Clinic and Thomas Jefferson University Hospital…
Editorial
With inflation hitting yet another record-breaking high—9.1% at the time of publication—hospitals and health systems continue to contend with challenging supply chain disruptions that force leaders into a constant state of putting out fires. Supply chain issues are not new. But instead of having time to plan ahead and implement…
Epic’s revenue grew 13%, hit $3.8B in 2021
Editor's Note The healthcare software company Epic has been seeing an upward trend in its revenue, reporting “double-digits [growth] every year for the last decade,” Becker’s Health IT June 13 reports. Citing CB Insights and Forbes, Becker’s noted that Epic’s revenue grew by 13% in 2021 and hit $3.8 billion.…
Effectiveness of decontaminating PPE with methylene blue
Editor's Note This study led by researchers at the University of Washington, Seattle, finds that methylene blue with light (MBL) photochemical treatment can be used to decontaminate personal protective equipment (PPE) contaminated with COVID-19. MBL robustly and consistently inactivated three coronaviruses, including COVID-19, with 99.8% to >99.9% virus inactivation across…
FDA: Class I recall of Getinge Flow-c, Flow-e Anesthesia Systems
Editor's Note The Food and Drug Administration (FDA) on July 6 identified the recall by Getinge USA Sales Inc of its Flow-c and Flow-e Anesthesia Systems as Class I, the most serious. The recall was initiated after reports of cracked or broken on/off switchs on the systems’ suction units. If…
Economic health of hospitals looks to be improving
Editor's Note According to a report from the Institute for Supply Management (ISM), economic activity in the hospital subsector grew in June for the 25th consecutive month, Healthcare Purchasing News July 7 reports. Following are some observations, according to Nancy LeMaster, MBA, ISM chair for the Hospital Business Survey Committee:…
Improving endoscope precleaning workflows
Editor's Note Researchers at the Memorial Sloan Kettering Cancer Center, New York City, gathered an interdisciplinary team to organize a safe and time efficient precleaning workflow for their fast-paced GI clinic that decreased time spent precleaning by 50%. The researchers identified inadequate precleaning processes, including staff who did not follow…
The Joint Commission issues new Quick Safety on managing packaged sterile supplies, devices
Editor's Note The Joint Commission, on June 28, announced a new Quick Safety advisory on “Managing packaged sterile supplies and devices.” The advisory provides guidance to keep patients safe from infection and harm from expired or compromised supplies and devices and urges healthcare workers (HCWs) to pay close attention to…
FDA: Class I recall of GE Healthcare’s CARESCAPE R860 Ventilator
Editor's Note The Food and Drug Administration (FDA) on June 28 identified the recall by GE Healthcare of its CARESCAPE R860 Ventilator as Class I, the most serious. The recall was initiated because the ventilator backup batteries, including replacement backup batteries, may run out before they are expected to do…

 Free Daily News
Free Daily News