 Supply Chain/Technology
Supply Chain/Technology
Gene-edited pig kidney, heart pump combined in transplant surgery milestone

Editor's Note The second-ever living recipient of a gene-edited pig kidney was the first to have the new organ paired with a mechanical heart pump, according to an April 24 CNN report. The subject, 54-year-old Lisa Pisano, underwent the milestone surgery at NYU Langone Health. She had heart failure and…
Catheter sterility concerns prompt Class 1 FDA recall for surgery trays

Editor's Note The US Food and Drug Administration has designated DeRoyal Industries’ recall of GeoMed custom tracecarts a class 1, the most serious type of recall indicating a risk of serious injury or death. According to the April 24 FDA notice, the recall is due to sterility concerns with the…
Large amounts of personal data possibly stolen in Change Healthcare cyberattack

Editor's Note Personal information about a “substantial portion of people in America” could be at risk from the February 21 cyberattack on UnitedHealth’s Change Healthcare division, The Associated Press reported April 23. Although the company reports no signs of full medical histories or charts were released, notifying all who were…
AI model helps predict patient decline, drive collaborative care

Editor's Note An AI prediction model that uses near-real-time data to generate a patient risk score shows the promise of AI for helping physicians and nurses coordinate on patient care, according to findings published March 25 in JAMA Internal Medicine. Performed by researchers at Stanford Medicine, the study examined an…
Supply chain stability improves, but threats remain
Editor's Note Released April 9 by the Association for Supply Chain Management (ASCM) and KPMG LLP, the KPMG Supply Chain Stability Index reveals an overall improvement in supply chain stability throughout 2023. However, points of weakness persist. According to the index, factors preventing a complete return to pre-pandemic normalcy include the…
FDA approves imaging drug for detecting cancer after lumpectomy

Editor's Note The US Food and Drug Administration (FDA) approved Lumisight (pegulicianine), a fluorescent imaging drug used to detect cancerous tissue during lumpectomy, on April 17. Administered intravenously prior to surgery, Lumisight is designed for use with the Lumicell Direct Visualization System (DVS) or another imaging device that is FDA-approved…
Penn Medicine anesthesia, waste initiatives boost OR sustainability

Editor's Note Penn Medicine has made significant strides in reducing the environmental footprint of the OR through department- and team-level initiatives, according to a March 29 report in Penn Medicine news. Driven by CIRCE: Medicine, a faculty group consisting of providers from Penn Medicine and Children’s Hospital of Philadelphia, examples…
Surface disinfection: How to play your cards right with UVC light
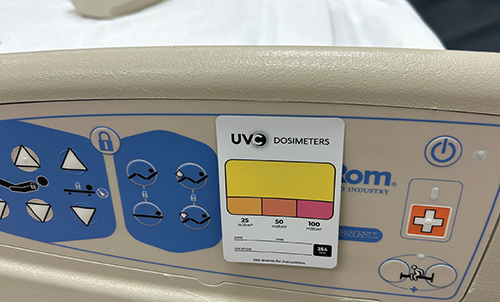
Approximately one in 31 hospital patients has at least one infection on any given day, according to the Centers for Disease Control and Prevention (CDC). In surgical settings, the risk is even higher, with up to 7% of patients developing an infection during surgery. These infections can lead to a…
Unveiling ECRI’s 2024 Top 10 Patient Safety Concerns list
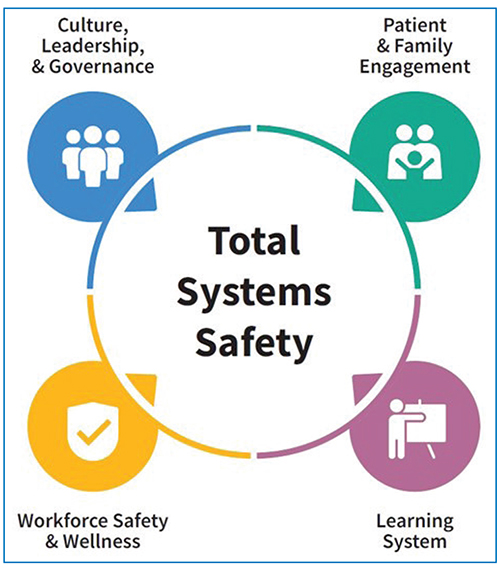
From integrating new technology to navigating shifts in care delivery and mitigating burnout, the most pressing challenges for healthcare organizations tend to be multifaceted problems that demand multifaceted solutions. For evidence of that, look no further than the Top 10 Patient Safety Concerns 2024 list from ECRI. For every risk…
Survey assesses continued impact of Change Healthcare cyberattack

Editor's Note Fallout from the February 21 cyberattack on Change Healthcare continues to threaten physician practices and their patients nationwide, with respondents to a recent American Medical Association (AMA) survey indicating difficulties with insurance claims and eligibility verification. AMA published the results of the informal survey April 10. Conducted March…

 Free Daily News
Free Daily News