 Regulations/Legal
Regulations/Legal
Patient falls, wrong surgeries, care delays lead rise in sentinel events

Editor's Note Sentinel events reported to The Joint Commission increased by 12% in 2024, with patient falls once again leading the list of serious adverse events followed by wrong surgeries. The Joint Commission released the data in an annual review July 9. Wrong surgery events increased 13% from the…
Researchers link full practice laws for APRNs to better state health rankings

Editor's Note States that grant full practice authority to advanced practice registered nurses (APRNs) rank significantly higher in health system performance than those that impose physician supervision requirements, according to a July 3 report from the University of Missouri. The article focuses on a study led by researchers at the…
FDA inspections slow as support staff layoffs strain overseas oversight

Editor's Note Logistical staff layoffs at the US Food and Drug Administration (FDA) are hindering the agency’s ability to scrutinize drug manufacturing safety in foreign countries, according to a July 7 report in ProPublica. A spokesperson from the US Department of Health and Human Services (HHS) told ProPublica that FDA…
FDA early alerts flag pump controllers, software

Editor's Note Recent early alerts from the US Food and Drug Administration (FDA), issued when the agency becomes aware of potentially high-risk issues, involve Abiomed’s Automated Impella Controller (AIC) and infusion pump software from Baxter. The AIC system, which is the user control interface for the Impella catheter blood pump,…
Study: Racial, insurance disparities persist in access to buprenorphine after opioid-related events

Editor's Note Black and Hispanic patients remain significantly less likely than White patients to receive buprenorphine after an opioid-related health care event, according research published June 26 in JAMA Network Open. Patients with Medicaid or Medicare Advantage also had higher odds of receiving buprenorphine than those with commercial insurance. The…
Oregon tightens control on physician practice ownership, indirectly impacting office-based surgery

Editor's Note Oregon has enacted the nation’s strictest law yet to curb corporate control of physician practices, a move that could indirectly affect office-based surgery (OBS) centers structured as medical clinics. As reported by Modern Healthcare on June 13, the new law reinforces the state’s corporate practice of medicine doctrine…
Mobile ORs can change the game, bridge gaps in surgical care
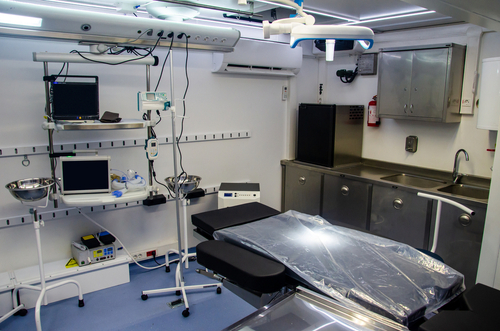
Imagine an innovative, safe, and highly efficient OR not confined by walls but on wheels—crossing rugged terrains, bustling cities, and disaster-stricken areas to deliver life-saving surgical care in underserved areas. That is the premise and promise of mobile ORs. They are not just mobile units. With some of the technological…
ASC adaptability depends on properly structured leadership

Takeaways • Different types of ASC leadership structures can be adapted to meet organizational needs. • Regulations, accreditation standards, size, and ownership types are examples of factors influencing the leadership structure. • Ongoing success of the ASC leadership team depends on factors such as governing body diversity and strategic planning.…
FDA designates Class 1 recall for angiographic catheter

Editor's Note The US Food and Drug Administration (FDA) has designated Cook’s recent recall of the Beacon Tip 5.0 Fr Angiographic Catheter as a Class 1, the most severe category indicating serious risk of injury or death. The recall was reportedly motivated by reports of tip separation both prior to…
The Joint Commission announces accreditation overhaul

Editor's Note The Joint Commission has launched a major redesign of its healthcare accreditation and certification programs with Accreditation 360: The New Standard. According to a June 30 announcement, the new framework introduces outcome-focused performance tools, eliminates hundreds of requirements, and promises to made standards publicly accessible. Reportedly supported by…

 Free Daily News
Free Daily News