Most OR teams would never suspect that their trusted surgical lights and warming systems might be undermining the sterility they work so hard to protect. Yet that’s exactly what Baxter’s recent research suggests, and the implications may compromise your OR’s ability to maintain sterility and protect both surgical teams and patients.
Ask most clinicians about infection prevention, and you’ll hear about proper sterilization and effective ventilation systems. But what’s rarely discussed is how surgical lights and other equipment positioned over the patient can unintentionally interfere with the ventilation airflow.”
Traditional surgical lights, for example, can block airflow, reduce velocity, and even create stagnant “recirculation zones” where bacteria-carrying particles (BCPs) linger.1 Likewise, forced air warming (FAW) systems can disrupt laminar flow, pushing potentially contaminated air upwards into sterile zones. 2 Together, these disruptions may elevate the risk of surgical site infections (SSIs) 3, despite surgical teams doing everything “by the book”.
At the iExcel simulation center at the University of Nebraska Medical Center, Baxter researchers recreated a working OR to test how different equipment setups affected airflow and surgical smoke dispersal. Two scenarios were compared:
Scenario A: A surgical light designed with an airflow cut-out paired with conductive (Air-free) warming
Scenario B: A traditional surgical light without a cut-out paired with FAW
The results? Astounding.
With scenario A, airflow remained smooth and surgical smoke was pushed out of the sterile field, giving surgeons better visualization and supporting sterility.5 With scenario B, airflow stalled, smoke lingered over the surgical site, and contaminants had greater opportunity to settle where they shouldn’t.
Why does this matter beyond the research setting? Because real-world ORs face the same challenges every day. The cumulative impact of traditional lights and FAW systems isn’t always visible – until it’s too late.
These are the facts:
“Researchers calculated that the average daily [surgical smoke] exposure for perioperative personnel had the passive effects of smoking 27 to 30 unfiltered cigarettes per day?” 9
In short, optimal OR airflow isn’t just a “nice to have”, it’s a safety issue and soon, a potential compliance issue.
Surgical lights with innovative airflow cut-outs – sometimes designed with a clover-shaped head – can help prevent the formation of low-velocity “dead zones”. Instead of trapping BCPs, they support ventilation systems doing their job, carrying contaminants away from the sterile field. 5
Patient warming systems also play a role. Conductive “air-free” warming solutions, help clinicians maintain patient normothermia without sending warm air currents upward. FAW systems on the other hand, may push plumes of air – and whatever’s in them – into sterile areas. 2
These aren’t just subtle engineering differences; they’re differences that can influence sterility across the OR.
Let’s break it down:
| Traditional Surgical Lights | Surgical Lights with Airflow Cut-Outs |
| Without any openings for air to flow through these lights, ventilation flow is blocked, and stagnant zones are created where contaminants can settle. 1 | The cut-out design on these surgical lights can help support ventilation flow, reduce “dead zones”, and help surgical smoke disperse efficiently. 5 |
| Forced Air Warming (FAW) | Conductive (Air-Free) Warming |
| With warm air being blown through an external blower, warm air can be pushed upwards and potentially disrupt the dispersal of surgical smoke. 2 | Since patients are warmed conductively without the need for external blowers, you can keep them warm without introducing disruptive air currents. 5 |
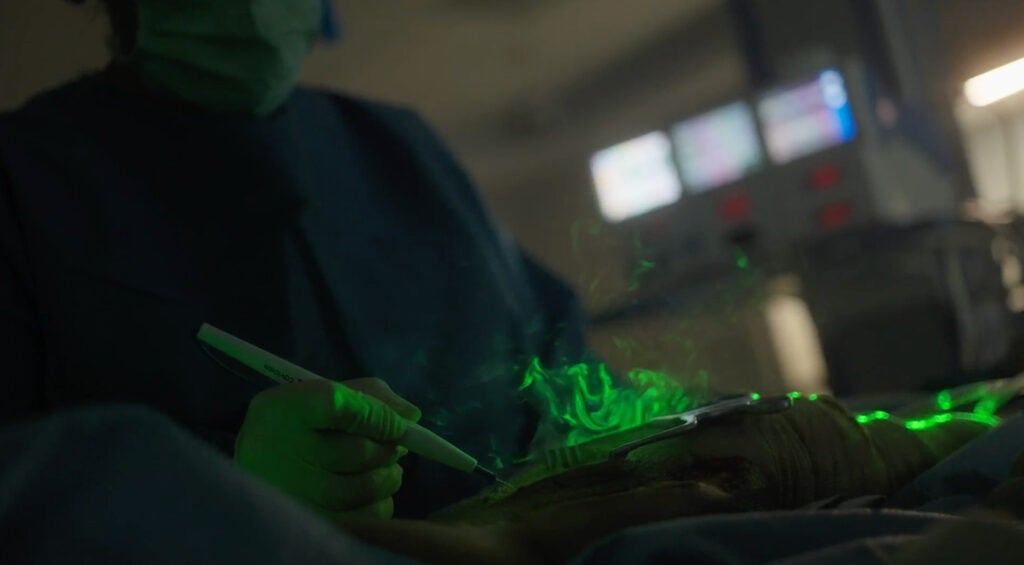
New findings from a recent study conducted in 2022 reveal that ignoring airflow issues has deeper consequences than many surgical teams may realize. Surgical smoke is more than a nuisance – it’s a carrier of chemical compounds like benzene and toluene, as well as biological material including viruses and bacteria. 7 Over time, these contaminants don’t simply breakdown and go away. They can accumulate, cling to surfaces, and even be absorbed by both surgical teams and patients during procedures.
“Up to 90% of surgical smoke particles are <2.5 µm—small enough to bypass standard mask filtration.” 4
Here’s the uncomfortable truth: Ignoring these findings may be overlooking one of the easiest ways to improve safety in the OR. You might also risk non-compliance with emerging smoke evacuation requirements – or miss opportunities to protect surgical staff from inhaling toxic surgical plumes.
Optimizing OR airflow isn’t just about upgrading HVAC systems – it’s about looking at every factor that shapes how air moves in the operating room. From the lights overhead to the warming system under the drapes, these decisions can have ripple effects on infection prevention, staff safety, and the health of everyone in the room.
This blog is just the beginning. The whitepaper dives into:
Don’t wait until your team is facing a smoke-filled field or you’re out of compliance. Explore Baxter’s research into airflow-friendly OR design and potential strategies to support infection prevention.
References
Baxtor.com
US-CS388-250009 (v1.0) 09/2025