
Editor's Note The ECRI Institute on August 23 announced a new free white paper, “Value Analysis: Best Practices for Navigating the Evidence Maze,” to help value analysis committees bring an objective, systematic approach to the decision-making process for health technologies and interventions. The white paper includes three case studies that…
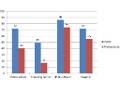
Editor's Note Results of a new national survey by the Hartford Consensus about views of the public and healthcare professionals on active shooter events in hospitals show that: 61% of the public and 62% of professionals believe physicians and nurses have a special duty to protect patients during an active…

Editor's Note The Joint Commission announced August 21 that Standard PI.02.01.03: “The hospital improves its performance on ORYX accountability measures” and the Standard's one Element of Performance: “The hospital achieves a composite performance rate of at least 85% on the ORYX accountability measures transmitted to the Joint Commission” will be…
Editor's Note The Food and Drug Administration (FDA) on August 18 announced the recall by Vital Rx, dba Atlantic Pharmacy and Compounding (Pompano Beach, Florida) of all lots of compounded injectable medications because of sterility assurance. During a recent FDA inspection, investigators observed unsanitary conditions, including poor sterile production practices.…

Editor's Note The Veterans Affairs Medical Center, in Buffalo, New York, is notifying 526 patients that they may be at risk of infection from improperly cleaned endoscopes, the August 16 Buffalo News reports. A recent hospital review of endoscope disinfection processes found that steps in the manufacturer’s instructions may not…

Editor's Note ChartLogic (Salt Lake City) is reporting that as of August 9, 2017, a total of 419 ICD-10 codes were added, 273 codes were revised, and 123 codes were being deleted, according to the August 15 Becker’s Hospital CFO Report. The changes are slated for implementation on October 1,…
Editor's Note The Food and Drug Administration on August 18 announced the recall by Bella Pharmaceuticals (Chicago, Illinois) of all lots of unexpired sterile drug products because of a lack of sterility assurance. Affected products include all lots distributed April 17, 2017, to August 10, 2017, nationwide. Products are packaged in…
Editor's Note This study from the Joint Commission finds that criteria for assessing whether outcome measures are accurate and valid enough to use for public reporting, payment, and accreditation are not well-defined. The authors propose four criteria to assess outcome measures: Strong evidence should exist that good medical care leads…
Editor's Note The Food and Drug Administration (FDA) on August 16 classified the recall by Cook Medical Inc (Bloomington, Indiana) of its Zenith Alpha Thoracic Endovascular Graft as Class I, the most serious. Cook Medical is aware of reported cases where the graft became blocked or closed with blood clots…

Editor's Note In this multicenter study, on-pump coronary artery bypass grafting (CABG) led to significantly higher rates of 5-year survival and event-free survival than off-pump CABG. From 2002 to 2007, a total of 2,203 patients at 18 medical centers were randomly assigned to undergo either on-pump (1,099 patients) or off-…