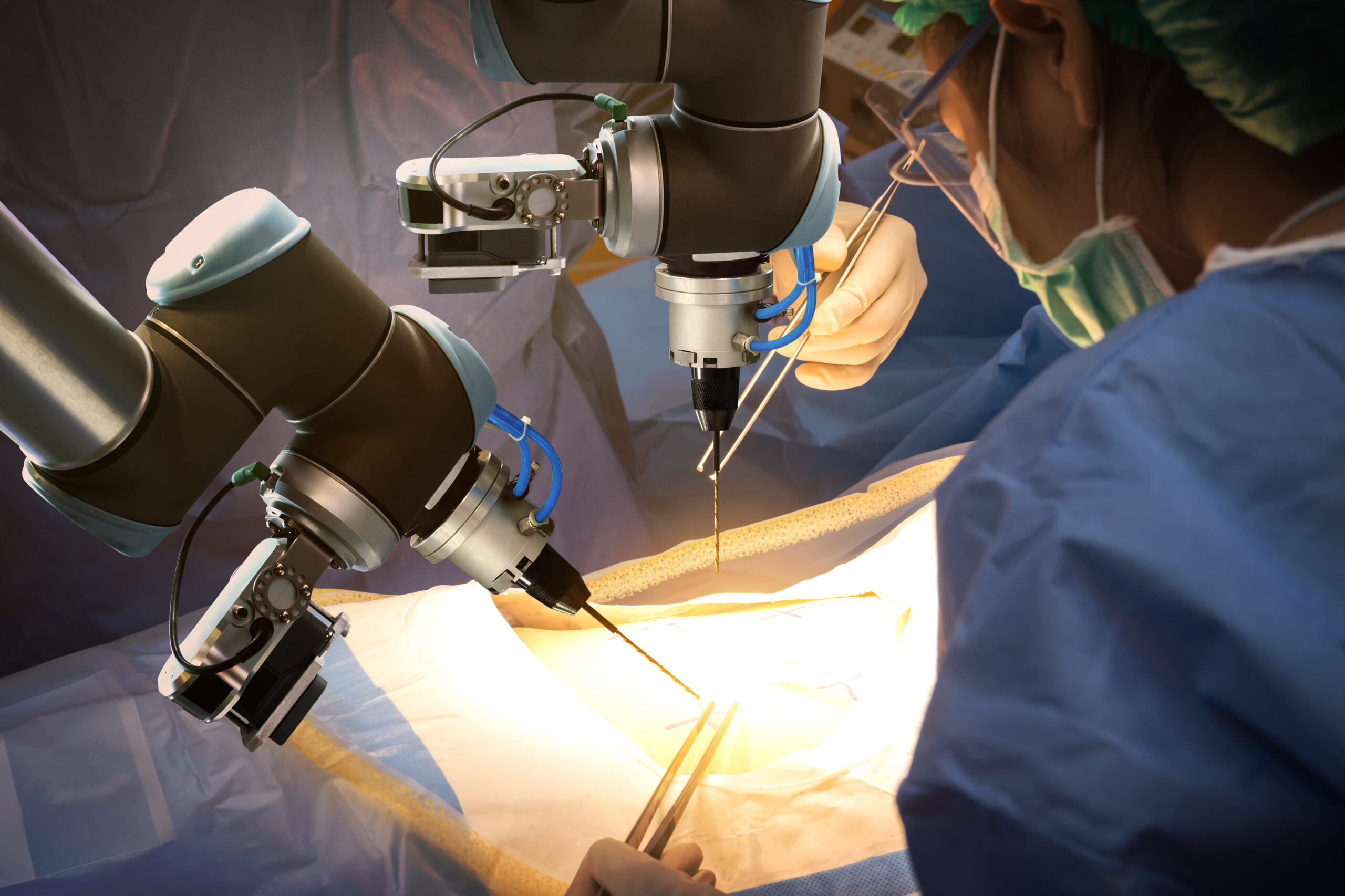
The St Elizabeth Healthcare organization in northern Kentucky has eight facilities, including two surgery centers. The flagship hospital on the Edgewood campus has 534 beds and 22 ORs and typically performs some 14,000 surgical procedures per year. Robots help manage the volume. The Edgewood, Florence, and Ft Thomas hospitals have…

Consider the following requests posed to OR scheduling staff by surgeons: • “I need two blocks in different rooms to accommodate my daily case volume.” • “Can’t I just use that empty room to start my next case while the first case is wrapping up?” Surgeon requests like these are…

David Shapiro, MD, who is a risk manager/medical director at several ambulatory surgery centers (ASCs) near Tallahassee, Florida, has seen his fair share of contracts with anesthesia services. Navigating these types of contracts at a time when anesthesiology is suffering from a workforce shortage and high costs for services is…
Editor's Note In this study of Medicare patients, researchers from the University of Michigan, Ann Arbor, find that loneliness is associated with an increased risk of death at 30 days after nonelective surgery. Of 4,453 patients in the analysis, 623 (14%) had nonelective surgery. The mean loneliness score was 1.5. Unadjusted…
Editor's Note This study from the University of Maryland, Baltimore, finds that full and partial COVID-19 vaccination reduced postoperative complications. Researchers analyzed data from patients having surgery at 1,283 VA medical facilities nationwide. Of 87,073 surgical patients, 20% were fully vaccinated, 15% were partially vaccinated, and 65% were unvaccinated. Among…
Editor's Note The Food and Drug Administration (FDA) on November 1 identified the recall of Teleflex’s Iso-Gard Filter S as Class I, the most serious. Iso-Gard filters are breathing circuit bacterial filters that are connected to respiratory equipment or breathing systems in operating rooms and ICUs. The recall was initiated…
Editor's Note This study from the University of California San Francisco finds that pediatric patients with risk factors for respiratory complications had a greater incidence of adverse events under general anesthesia during periods of unhealthy air quality caused by wildfire smoke. A total of 625 pediatric patients were included in…
Editor's Note The Food and Drug Administration (FDA) on October 31 issued a Medical Device Safety Communication on tracheostomy tube shortages. Included in the shortages are Bivona tracheostomy tubes manufactured by ICU Medical, which are more likely to affect pediatric patients. Also on the shortage list are JOH Tube Tracheostomy…
Editor's Note As of November 1, the Food and Drug Administration (FDA) listed 26 amoxicillin products on its current drug shortages site. The FDA says increased demand is the largest cause of the shortage. The FDA does not list resupply dates, but the American Society of Health-System Pharmacists (ASHP) estimates…
Editor's Note Reducing noise in the OR positively affects children’s postoperative behavior, according to research presented October 24 at the Anesthesiology 2022 annual meeting in New Orleans. The study, led by researchers at Nationwide Children’s Hospital, Columbus, Ohio, included 64 preschool children (ages 4 to 5 years old) having general…