AAMI releases updated protective barriers standard
 The Association for the Advancement of Medical Instrumentation (AAMI) has revised the ANSI/AAMI PB70 Liquid barrier performance and classification of protective apparel and drapes intended for use in healthcare facilities.
The Association for the Advancement of Medical Instrumentation (AAMI) has revised the ANSI/AAMI PB70 Liquid barrier performance and classification of protective apparel and drapes intended for use in healthcare facilities.
This revised standard provides healthcare professionals with a better understanding of the barrier protection properties of available protective apparel. The standard also provides guidance to manufacturers as they design drapes and protective apparel. It establishes the minimum barrier performance requirements, classification system, and associated labeling requirements for protective apparel, surgical drapes, and drape accessories intended for use in healthcare facilities.
This standard provides four classification levels of barrier performance, details the specific barrier requirements for each level, and includes new apparel that can be used as protective barriers in healthcare settings. The higher level of classification, the higher the barrier protection level.
The revised PB70 now includes decontamination gowns, togas, hoods, headwear, and footwear (before the revision, there were no requirements for decontamination gowns).
A new requirement for gowns worn in the decontamination area is that they now be labeled as “intended for use in decontamination applications.” Their design and construction are based on the anticipated location of use and degree of liquid contact.
Critical zones are those where direct contact with blood, body fluids, or other potentially infectious materials are most likely to occur, and those zones must have a barrier performance of at least Level 3. The manufacturer must provide detailed information regarding the barrier performance of each critical zone.
It is important to note that attire worn in the decontamination area must also meet the requirements of the Occupational Safety and Health Administration’s (OSHA’s) Bloodborne Pathogen Standard. The OSHA standard states that the only attire that may be worn in the decontamination area is that which does not permit blood or potentially infectious materials to pass through and reach employees’ clothing. The standard does not address the properties of decontamination garments that protect against hazardous chemical agents used during medical device cleaning, disinfection, decontamination, or sterilization.
For hoods that consist of a head and neck covering, with or without an integrated face shield, the revised PB70 states that the critical zone covers the entire front of the hood assembly and extends past the face to the ears (but not over the ears) and has a minimum barrier protection of Level 1. The entire back of the hood, extending from the front of the ears to the back of the head, the top area of the hood, and any filters in this area are excluded from the critical zone. For specific information regarding the barrier performance of each critical zone component of a hood, healthcare providers may contact the manufacturer.
Footwear barrier performance should be at least Level 1, the revised standard states.
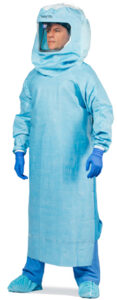
Togas provide superior protection because they combine a protective gown with an integrated hood and face shield. Unlike gowns, togas protect the neck. It is anticipated that togas will be used in procedures that typically involve a large amount of body fluids, such as orthopedic cases. Togas have critical zones that provide optimal protection, and these zones will be labeled separately and prominently with the class of barrier performance. (See Figure 1 for an example of a toga.)
When a healthcare worker selects protective apparel, the attire should be prominently labeled with its class of barrier performance. For the revised PB70, labeling requirements were added for each package containing protective gowns, such as decontamination gowns, to state that the gowns meet one of the following appropriate descriptions:
• full coverage gown
• non-protective back gown
• open-back gown.
Surgical gowns with back panels that do not meet at least the requirements for Level 1 barrier performance must be prominently labeled with a “Back Is Non-Protective” warning. Additionally, protective barriers are to be labeled with an expiration date to the lowest unit of packaging (eg, box or bag). Like other medical devices, the technical information is provided by the manufacturer upon request.
 —Susan Klacik, BS, FCS, ACE, CHL, CIS, CRCST, AAMIF, is clinical educator at Healthcare Sterile Processing Association (HSPA) and its representative to the Association of the Advancement of Medical Instrumentation (AAMI). Klacik has authored numerous articles and represents IAHCSMM for regulatory affairs dealing with sterilization and disinfection.
—Susan Klacik, BS, FCS, ACE, CHL, CIS, CRCST, AAMIF, is clinical educator at Healthcare Sterile Processing Association (HSPA) and its representative to the Association of the Advancement of Medical Instrumentation (AAMI). Klacik has authored numerous articles and represents IAHCSMM for regulatory affairs dealing with sterilization and disinfection.
This article has presented a high-level summary of additions to ANSI/AAMI PB70 that pertain to Sterile Processing professionals. The revised standard is available at www.aami.org.

 Free Daily News
Free Daily News