 Safety/Quality
Safety/Quality
Penn Medicine anesthesia, waste initiatives boost OR sustainability

Editor's Note Penn Medicine has made significant strides in reducing the environmental footprint of the OR through department- and team-level initiatives, according to a March 29 report in Penn Medicine news. Driven by CIRCE: Medicine, a faculty group consisting of providers from Penn Medicine and Children’s Hospital of Philadelphia, examples…
Managing challenging employees: How to help

TAKEAWAYS • Addressing an employee with a competency or behavior issue is important for the well-being of staff and managers. • Determining the reason for the issue is an important first step to resolving it. • Sources of support for managers include colleagues, human resources, and educators. In today’s perioperative…
Surface disinfection: How to play your cards right with UVC light
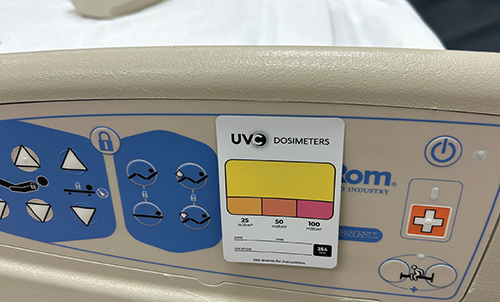
Approximately one in 31 hospital patients has at least one infection on any given day, according to the Centers for Disease Control and Prevention (CDC). In surgical settings, the risk is even higher, with up to 7% of patients developing an infection during surgery. These infections can lead to a…
Unveiling ECRI’s 2024 Top 10 Patient Safety Concerns list
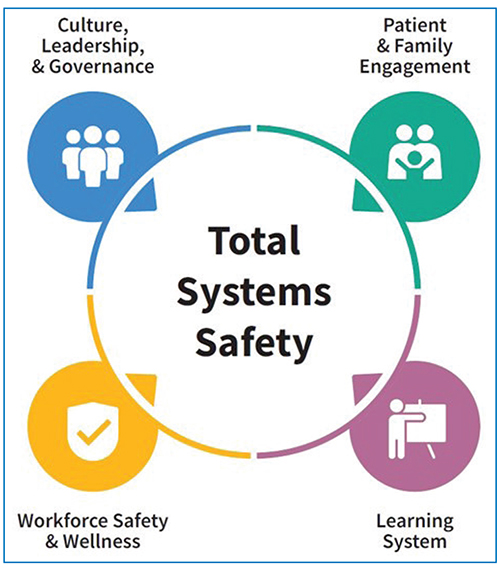
From integrating new technology to navigating shifts in care delivery and mitigating burnout, the most pressing challenges for healthcare organizations tend to be multifaceted problems that demand multifaceted solutions. For evidence of that, look no further than the Top 10 Patient Safety Concerns 2024 list from ECRI. For every risk…
Survey assesses continued impact of Change Healthcare cyberattack

Editor's Note Fallout from the February 21 cyberattack on Change Healthcare continues to threaten physician practices and their patients nationwide, with respondents to a recent American Medical Association (AMA) survey indicating difficulties with insurance claims and eligibility verification. AMA published the results of the informal survey April 10. Conducted March…
WHO sounds alarm on potential spread of bird flu

Editor's Note The World Health Organization (WHO) is sounding the alarm about the rise of the bird flu virus—H5N1—and the threat it poses to humans, Medical Xpress reported on April 18. Experts are concerned because the bird flu has recently spread from wild birds and poultry to cows and goats…
Neurosurgery trial: Early evacuation improves long-term hemorrhage outcomes

Editor's Note Medical management care with evacuation surgery could yield better 180-day outcomes than without in patents treated within 24 hours for acute intracerebral hemorrhage, according to study results covered in an April 10 MedPage Today report. The ENRICH (Early MiNimally-invasive Removal of IntraCerebral Hemorrhage (ICH)) trial is a multicenter,…
Drug shortages higher than ever

Editor's Note Shortages of active drugs in the US have reached a new record, according to an April 12 CNN report on data from the American Society of Health-System Pharmacists and the University of Utah Drug Information Service. The two organizations have been tracking this data since 2001, the CNN report says.…
FDA announces class 1 recalls for premixed embolic, infusion pump software

Editor's Note The US Food and Drug Administration has classified recalls of Boston Scientific’s Obsidio Conformable Embolic and Fresenius Kabi USA’s Ivenix Infusion System Large Volume Pump (LVP) software as class 1, the most serious category and an indicator of risk of serious injury or death. No injuries or deaths…
Propofol anesthesia for colonoscopy could help find polyps, prevent cancer

Editor's Note By putting the patient into deeper sedation during colonoscopy, propofol could help doctors find difficult-to-spot, potentially cancerous “serrated” polyps, according to a study published April 17 in Anesthesiology. As an alternative to moderate, “conscious” sedation, propofol facilitates a more thorough exam that is more likely to identify serrated…

 Free Daily News
Free Daily News