Editor's Note Updates to evidence-based guidance in the AORN 2026 Guideline for the Care and Cleaning of Surgical Instruments were summarized in a November 7 AORN news story to outline key updates such as new PPE recommendations for perioperative staff working in the decontamination area. For example, new recommendations in…
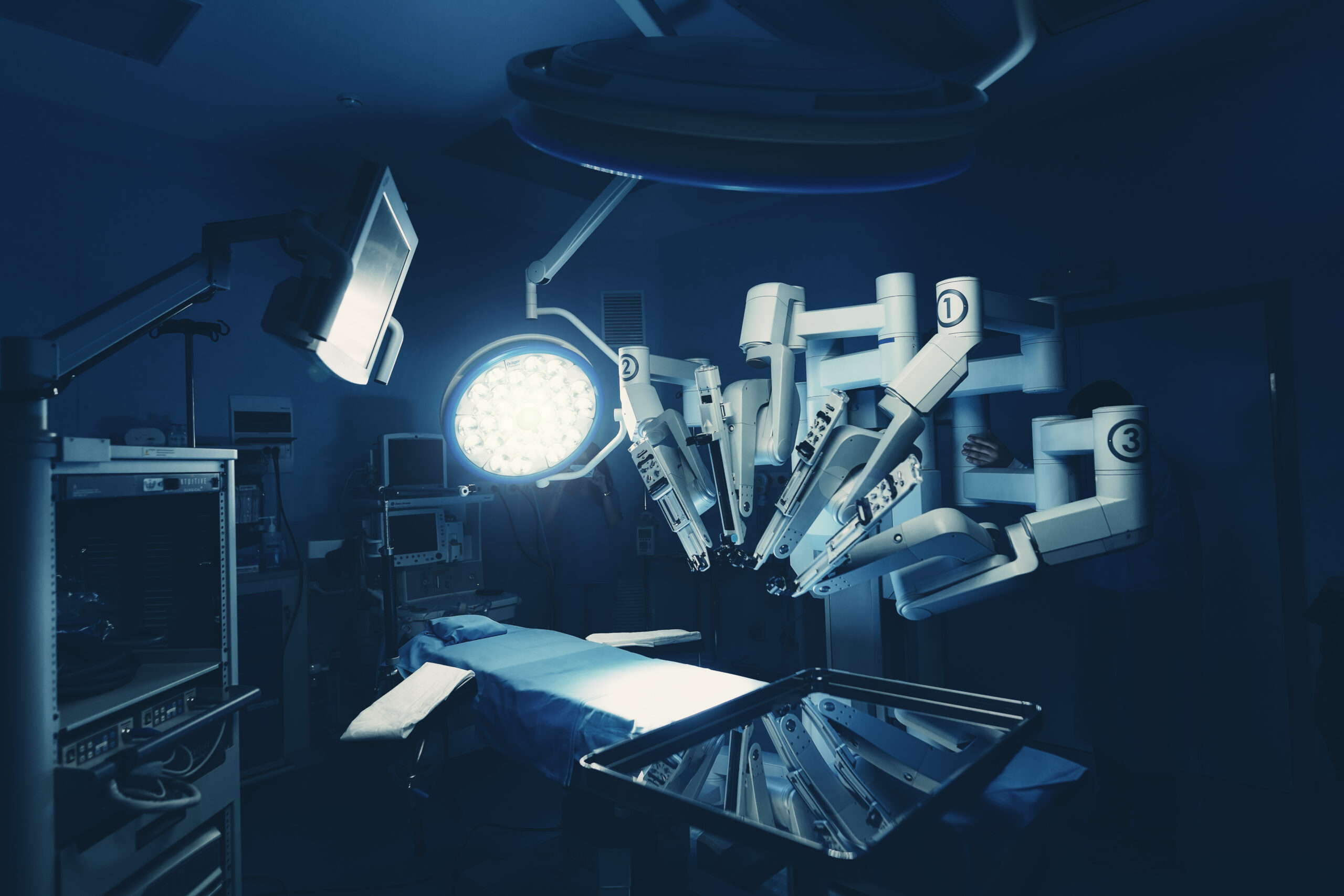
Takeaways • Determining the return on investment (ROI) for a robot-assisted surgery (RAS) program should include anticipated surgeon volume and past cases where RAS could have been used. • Some organizations dedicate staff to RAS cases, while others orient all to these procedures. • Offering ROI after hours require careful…

Editor's Note Surgical teams can dramatically reduce healthcare’s carbon footprint through waste reduction, energy efficiency, and smarter procurement, Cureus October 7 reports. The review’s authors describe surgery as both a major environmental challenge and a key opportunity for hospitals to align climate responsibility with clinical and financial goals. Healthcare contributes…

Editor's Note New and revised sterilization and reprocessing standards are reshaping the landscape for sterile processing departments, placing greater emphasis on chemical modalities, device-specific protocols, and system-wide quality management, according to the Healthcare Purchasing News May 27 update on compliance and standards. Among the most significant developments is the overhaul…
Preventing infection from contaminated surgical tools requires attention to every link in a complex chain of processes, from point-of-use pretreatment in the OR to the moment the freshly disinfected or sterilized item arrives at the next patient’s bedside. For those on the front lines, manufacturers’ written instructions for use (IFUs)…

In the OR, precision and focus can mean the difference between life and death. However, surgical patient outcomes hinge on more than the competence of those working in these inherently intense environments. Every procedure also depends on the laborious, behind-the-scenes efforts of the people responsible for ensuring every surgical instrument…

Reliable and robust enough for daily use on most medical devices, steam is the most common sterilant in healthcare facilities. However, using steam properly requires a balancing act. For example, too much moisture can lead to wet packs, while steam that is too dry might not be sufficient to achieve…

Editor's Note In 2023, hospitals and surgical centers saved more than $465 million and prevented nearly 98 million pounds of greenhouse gas emissions by utilizing reprocessed single-use medical devices, according to a survey by the Association of Medical Device Reprocessors (AMDR). DotMed reported the news October 21. The data indicate…

Editor’s Note The Association for Professionals in Infection Control and Epidemiology (APIC) is advocating for clearer reprocessing instructions for medical devices to improve patient safety and efficiency, Outpatient Surgery Magazine August 19 reports. Many current instructions for use (IFUs) are considered overly complex, outdated, and difficult to interpret, especially for…

Reforming instrument reprocessing practices does not always end with the main sterile processing department (SPD). Holding clinics to the same standard adds to the challenge, whether they are associated with hospitals or operate independently. Nonetheless, standardization is just as essential to maintaining efficiency and quality standards. Establishing and maintaining best…