Editor's Note Prescribing fewer opioid tablets postoperatively was associated with lower opioid consumption after anterior cruciate ligament reconstruction, and preoperative education was associated with lower duration and quantity of postoperative opioid use, this study finds. Of 264 patients analyzed, 109 received 50 tablets, 77 received 30 tablets and no education,…
Editor's Note A new scoring system reveals a strong agreement between patient-reported and physician-reported outcomes after surgery, the Mayo Clinic reports. The study enrolled 100 patients who had elbow or shoulder surgery. The average time between surgery and follow-up was 31 months. In the categorical ratings, patients and physicians agreed…
Editor's Note A visible-light continuous environmental disinfection (CED) system, used with manual cleaning, resulted in a significant reduction in microbial surface contamination and surgical site infections (SSIs) in an orthopedic OR, in this study. Samples were taken from 25 surfaces within two contiguous ORs sharing an air supply after manual…
Editor's Note Patients whose surgeons had more coworker reports about unprofessional behavior in the 36 months before their surgical procedures had a significantly increased risk of complications, this study finds. In this analysis of 13,653 patients having surgical procedures performed by 202 surgeons in two academic medical centers, 1,583 (11.6%)…
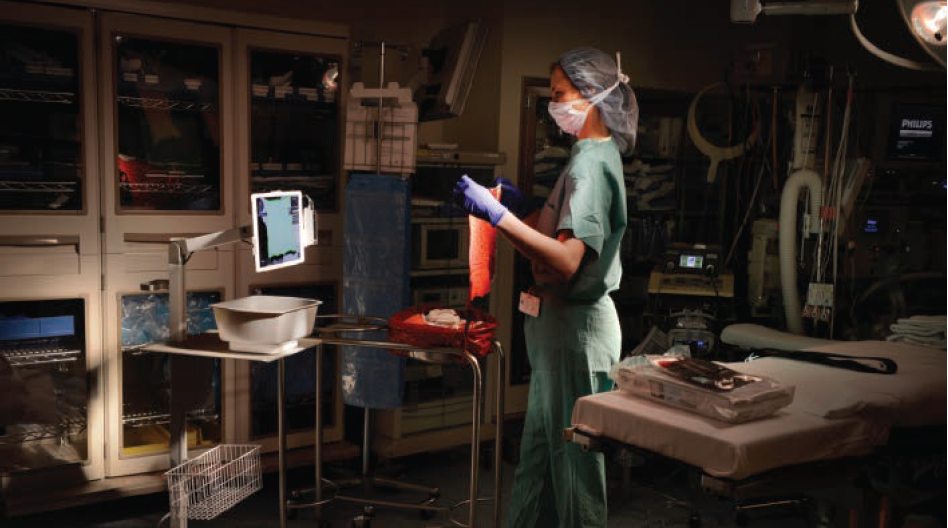
Blood loss during labor and delivery (L&D) and surgical procedures can lead to serious complications that might be prevented with early detection; however, detection can be challenging. For example, clinicians have traditionally estimated blood loss visually—a subjective and often inaccurate process. Humans’ eyes simply aren’t good at making precise measurements,…
Editor's Note In this study, patients who had transcatheter aortic valve replacement (TAVR) for bicuspid compared with tricuspid aortic stenosis had no significant difference in mortality, but they had a 30-day increased risk of stroke. In this cohort of 2,691 matched pairs of patients having TAVR for bicuspid vs tricuspid…
Editor's Note The majority of differences in outcomes between new and experiences surgeons are associated with the context in which care is delivered and patient complexity, rather than inexperience, this study finds. A total of 694,165 Medicare patients treated by 8,503 experienced surgeons were matched to 68,036 treated by 2,119…
Editor's Note The American College of Surgeons on June 6 called for interested hospitals to join a new cohort of the Agency for Healthcare Research and Quality (AHRQ) Safety Program for Improving Surgical Care and Recovery (ISCR). In the program, presented in collaboration with the Johns Hopkins Medicine Armstrong Institute…
Editor's Note The opioid epidemic has expanded the pool of overdose-death donors (ODDs) for heart transplantation. Even though ODDs have higher rates of hepatitis C, cardiac allograft quality indices are favorable, and recipient outcomes are similar to those with non-ODD hearts, this study finds. Of 15,904 heart transplant donors analyzed,…
Editor's Note Multimodal analgesia with minimal opiates improved pain control and significantly decreased opiate use and opiate-related adverse effects after discharge from elective total hip replacement surgery, finds this study. The 235 patients analyzed received one of three discharge pain regimens: Group A: scheduled-dose multimodal analgesia (ie, acetaminophen, meloxicam, gabapentin)…