
Editor's Note Nurses in Kansas are raising alarms that the state’s licensing system is punishing them for minor mistakes and lapses, leaving many unable to work, according to a series of reports by KWCH between August 27 and September 12. Lawmakers are now pressing the Kansas State Board of Nursing…

Editor's Note Artificial intelligence use in healthcare is accelerating, and the American Medical Association (AMA) is pressing health systems to establish clear governance policies before the technology outpaces oversight. Nearly 70% of physicians reported using artificial intelligence tools in 2024, a sharp rise from 38% in 2023, AMA News Wire…

Editor's Note Hospitals will soon face stronger accountability for suicide prevention, as The Joint Commission prepares to implement “National Performance Goal (NPG) 8” on January 1, 2026, its September 3 news update reports. The goal, titled “The hospital reduces the risk for suicide,” replaces current requirements under “National Patient Safety…

Editor's Note The Centers for Medicare & Medicaid Services (CMS) is moving to eliminate its Inpatient Only (IPO) List over the next 3 years, a decision that could permanently shift more surgical procedures from hospitals to outpatient settings. According to an August 24 article from Fierce Healthcare, the policy promises…
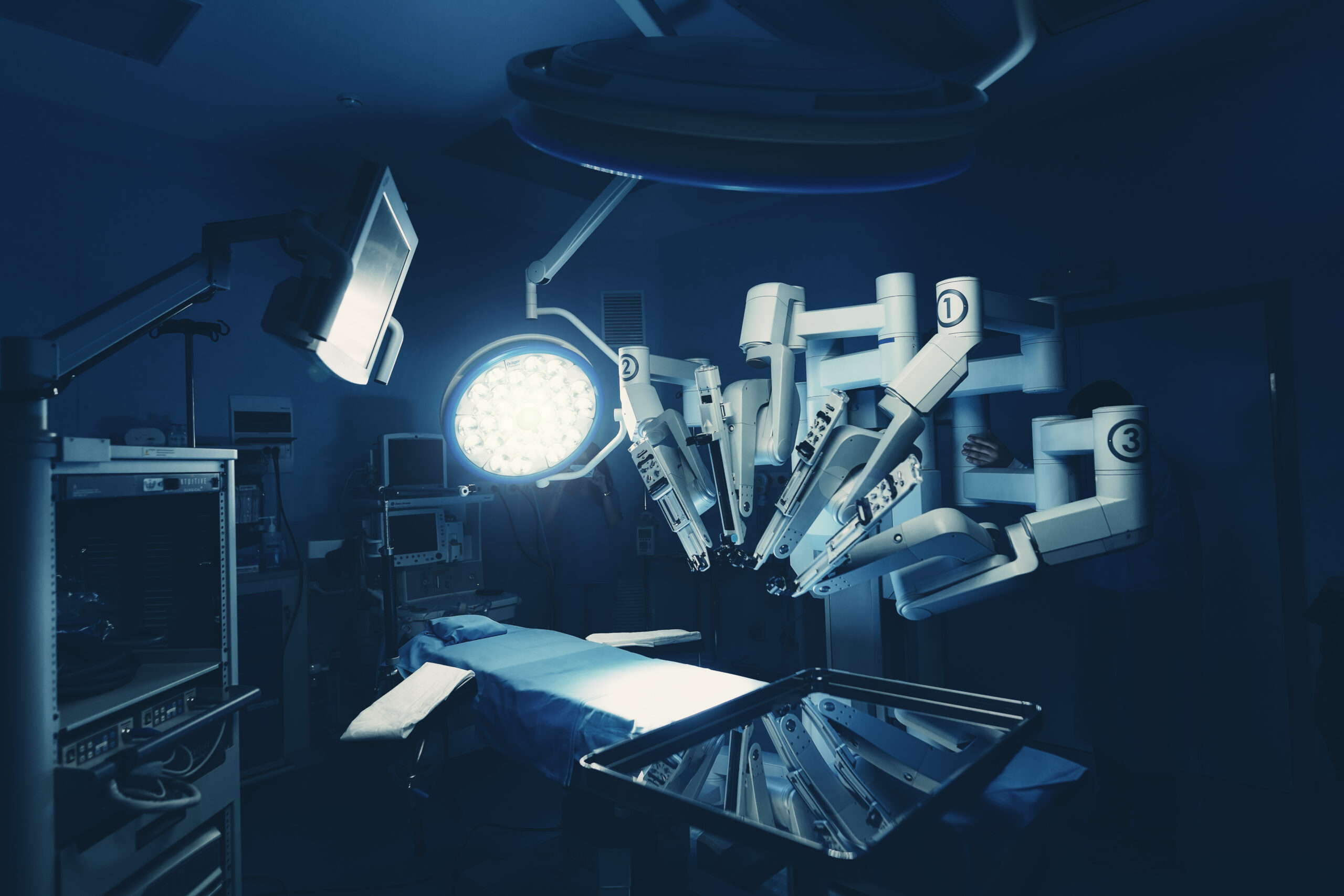
Takeaways • Robot-assisted surgery (RAS) is now an option in many specialties and for adult and pediatric patients; RAS-related ethical issues include access and patient privacy. • Intuitive Surgical continues to dominate the marketplace, but many companies are working on expanding their range of options. • Some current trends and…

Know the law. It could save your patients’ lives and your staff members’ practices. This is the advice of several medical legal experts to OR leaders to prevent lapses in patient protections that risk injury or even death. Beyond ensuring safe surgery and preventing litigation, perioperative nursing experts who advance…

When Phyllis Quinlan, PhD, RN, NPD‑BC, founded MFW Consultants in 1994, she was already on her third career pivot. The former social‑work intern turned emergency‑trauma nurse discovered that the high-stakes of the emergency department (ED), coupled with her human‑behavior insights from sociology and psychology degrees, gave her a rare vantage point on how…

Editor's Note The proposal from the Centers for Medicare & Medicaid Services (CMS) to eliminate the Medicare Inpatient Only (IPO) list over 3 years could significantly expand opportunities for ambulatory surgery centers (ASCs), with rural facilities among those positioned to benefit most, Ambulatory Surgery Center News August 12 reports. The…

Editor's Note The Accreditation Association for Ambulatory Health Care (AAAHC) released its v44 Standards, introducing new guidance designed to strengthen patient-focused care, streamline compliance, and align with evolving state regulations, an August 18 AAAHC announcement reports. As detailed in the news release, the v44 Standards, effective December 16, 2025, were…

Editor's Note Healthcare providers, payers, and analytics teams face sweeping ICD-10 changes this fall, with the 2026 code updates taking effect October 1, 2025, Wolters Kluwer July 14 reports. The release includes 614 new codes, 12 invalidations, 642 billability changes, 88 terminology revisions, and the creation of an entirely new…