
Editor's Note A federal shutdown has halted critical healthcare programs, disrupted Medicare telehealth and hospital-at-home coverage, and escalated partisan conflict over the Affordable Care Act (ACA) and Medicaid, multiple outlets report, including HealthLeaders October 1 and KFF Health News. The budget impasse reportedly is leaving both patients and providers in…

Editor's Note The Food and Drug Administration (FDA) has issued four medical device recalls and early alerts between September 23 and 30, covering Automated Impella Controllers, Olympus ViziShot 2 FLEX (19G) endoscopic aspiration needles, 3M Ranger blood and irrigation fluid warming systems, and BD Alaris infusion pump sets. The notices…

Editor's Note The plan to eliminate the Medicare Inpatient-Only (IPO) List and expand the Ambulatory Surgery Center Covered Procedures List (ASC-CPL) from the Centers for Medicare & Medicaid Services (CMS) has generated widespread reaction, with more than 3,900 comments submitted during the official feedback period, Ambulatory Surgery Center News September…

Editor's Note The Food and Drug Administration (FDA) has issued multiple high-risk medical device recalls in recent weeks, mid-September FDA announcements report. On August 21, Medline alerted customers that some of its convenience kits contain recalled Medtronic DLP Left Heart Vent Catheters. These catheters, used in cardiopulmonary bypass, may fail…

Editor's Note A new drug is giving surgeons a sharper view of cancer in the OR and helping preserve healthy tissue, Fast Company August 28 reports. Cytalux is an FDA-approved fluorescent agent that makes cancer cells glow green under infrared light, allowing surgeons to spot and remove malignant lesions with…

Editor's Note Dozens of companies are racing to stake a claim in the rapidly expanding surgical robotics market, with multiple launches, partnerships, and regulatory milestones signaling a pivotal moment for the field. Challengers to established leaders are advancing soft tissue systems, targeting specialty niches, and building executive teams to scale…

Editor's Note The Food and Drug Administration (FDA) has issued a series of early alerts this month regarding high-risk recalls from several leading medical device makers, citing patient safety risks ranging from pump failures to vascular complications. These alerts highlight issues with products from Johnson & Johnson’s (J&J’s) Abiomed unit,…
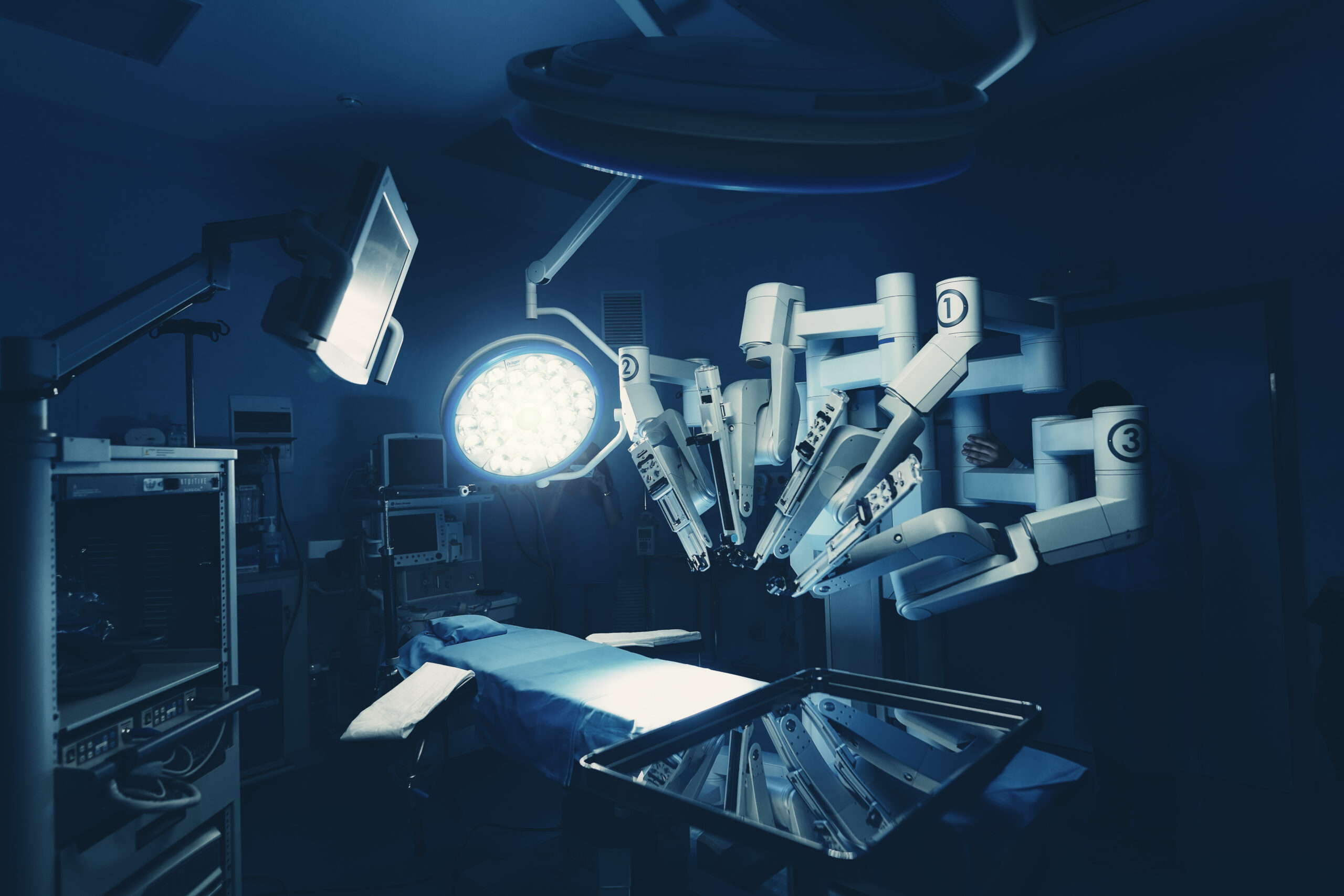
Takeaways • Robot-assisted surgery (RAS) is now an option in many specialties and for adult and pediatric patients; RAS-related ethical issues include access and patient privacy. • Intuitive Surgical continues to dominate the marketplace, but many companies are working on expanding their range of options. • Some current trends and…

Editor's Note The UC Davis Department of Orthopaedic Surgery has received more than $2.2 million in Department of Defense funding for two research projects addressing bone health in prostate cancer and preventing arthritis after joint injuries, a UC Davis Health July 15 news release reports. The larger grant, $1.8 million,…

Editor's Note The Food and Drug Administration (FDA) officially declared the nationwide shortage of sodium chloride 0.9% injection products over, saying a critical supply line for hospitals and surgical teams is restored. According to an August 8 statement from FDA Commissioner Martin A. Makary, MD, MPH, the resolution marks a…