
Editor's Note New and revised sterilization and reprocessing standards are reshaping the landscape for sterile processing departments, placing greater emphasis on chemical modalities, device-specific protocols, and system-wide quality management, according to the Healthcare Purchasing News May 27 update on compliance and standards. Among the most significant developments is the overhaul…
Preventing infection from contaminated surgical tools requires attention to every link in a complex chain of processes, from point-of-use pretreatment in the OR to the moment the freshly disinfected or sterilized item arrives at the next patient’s bedside. For those on the front lines, manufacturers’ written instructions for use (IFUs)…

Editor's Note Conventional cleaning protocols fail to remove visible soil and debris from lumened surgical instruments, raising urgent concerns about patient safety and sterilization efficacy. That’s the central finding of a study published February 11 in The American Journal of Infection Control, which used borescopes to inspect the lumens of…

Editor's Note High-level disinfection (HLD) fails to reliably eliminate harmful microbes from flexible endoscopes in real-world healthcare settings, according to a review of endoscope processing effectiveness published April 8 in the American Journal of Infection Control. The review highlights routine breaches in cleaning protocols and links contaminated endoscopes to numerous…

Editor's Note Every August, ambulatory surgery centers (ASCs) promote awareness of how they "have transformed the outpatient experience by offering a convenient and personalized alternative to the hospital outpatient surgical setting," notes the Ambulatory Surgery Center Association (ASCA). This year, ASCA and other organizations are using the month to educate…

Reforming instrument reprocessing practices does not always end with the main sterile processing department (SPD). Holding clinics to the same standard adds to the challenge, whether they are associated with hospitals or operate independently. Nonetheless, standardization is just as essential to maintaining efficiency and quality standards. Establishing and maintaining best…
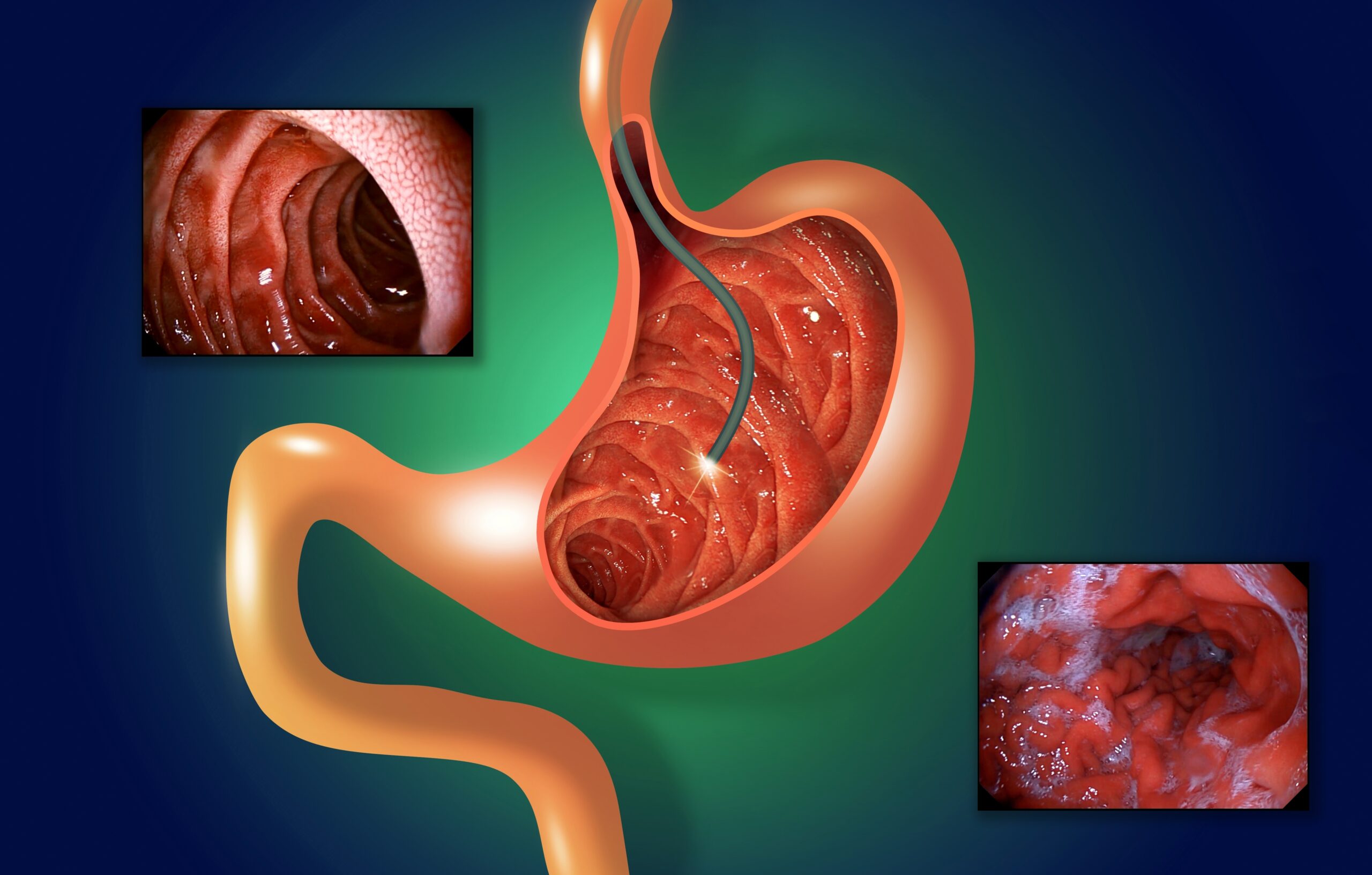
Gastrointestinal (GI) endoscopy is one of the most common procedures in the US. Performed more than 17.1 million times per year in inpatient and outpatient hospital settings as well as ambulatory surgery centers (ASCs), GI procedures account for 68% of all endoscopies, according to a May 2022 article in Digestive…
Inspecting surgical devices is a time-consuming process. However, diligently checking every instrument prior to sterilization is essential to ensuring safe, proper functioning. As the last people to see devices before they are used for patient care, sterile processing technicians must be thorough. Exterior surfaces should be inspected for flaws such…

Editor's Note A new guidance document covering the entire process for the selection, labeling, and sterile processing of dilators and ultrasound probes is available from The Association for the Advancement of Medical Instrumentation (AAMI). Released April 17, AAMI TIR99:2024; Processing Of Dilators, Transesophageal And Ultrasound Probes In Health Care Facilities…

Editor's Note Authors of a recent study evaluating the effectiveness of a forced-air drying system for endoscopes argue that the results reinforce the need to re-evaluate standard drying practices. Findings were published February 24 in the American Journal of Infection Control. Wet environments resulting from inadequate drying practices can result…