
Editor's Note The Food and Drug Administration (FDA) has issued multiple high-risk medical device recalls in recent weeks, mid-September FDA announcements report. On August 21, Medline alerted customers that some of its convenience kits contain recalled Medtronic DLP Left Heart Vent Catheters. These catheters, used in cardiopulmonary bypass, may fail…

The OR has a planned rhythm that relies on training, checklists, and teamwork to turn the complex surgical environment into an elegant orchestration that keeps patients safe. But efficiency and a climate of safety do not just happen—they depend on culture. When teams communicate openly, follow standards consistently, and feel…

Editor’s Note: This page is a companion piece to the main article, How nurse leaders can drive healthcare innovation: Countdown to the 2025 OR Manager Conference. Dan Weberg, PhD, MHI, RN, FAAN, will be attending this year’s OR Manager Conference for the first time. OR Manager talked to him about…

Much like the Blockbuster video store chain had a choice to innovate before the rise of streaming, Dan Weberg, PhD, MHI, RN, FAAN, thinks healthcare is at a similar crossroads. As an ER nurse turned educator, executive, and innovation expert, Dr Weberg sees the 4 million nurses nationwide as being…

Editor's Note The American College of Surgeons (ACS) has partnered with Lifesaving Technologies to expand access to emergency response equipment and training across the US, the ACS announced on September 3. The collaboration builds on the ACS Stop the Bleed program, which has already trained more than 5 million people…

Editor's Note Artificial intelligence use in healthcare is accelerating, and the American Medical Association (AMA) is pressing health systems to establish clear governance policies before the technology outpaces oversight. Nearly 70% of physicians reported using artificial intelligence tools in 2024, a sharp rise from 38% in 2023, AMA News Wire…

Editor's Note Artificial intelligence (AI) can track surgical performance with pinpoint accuracy, but true mastery still requires a human teacher, American Council on Science and Health August 20 reports. A randomized trial of an AI-powered surgical tutoring system found that while algorithms provided real-time error detection, the best learning happened…

Editor's Note Dozens of companies are racing to stake a claim in the rapidly expanding surgical robotics market, with multiple launches, partnerships, and regulatory milestones signaling a pivotal moment for the field. Challengers to established leaders are advancing soft tissue systems, targeting specialty niches, and building executive teams to scale…
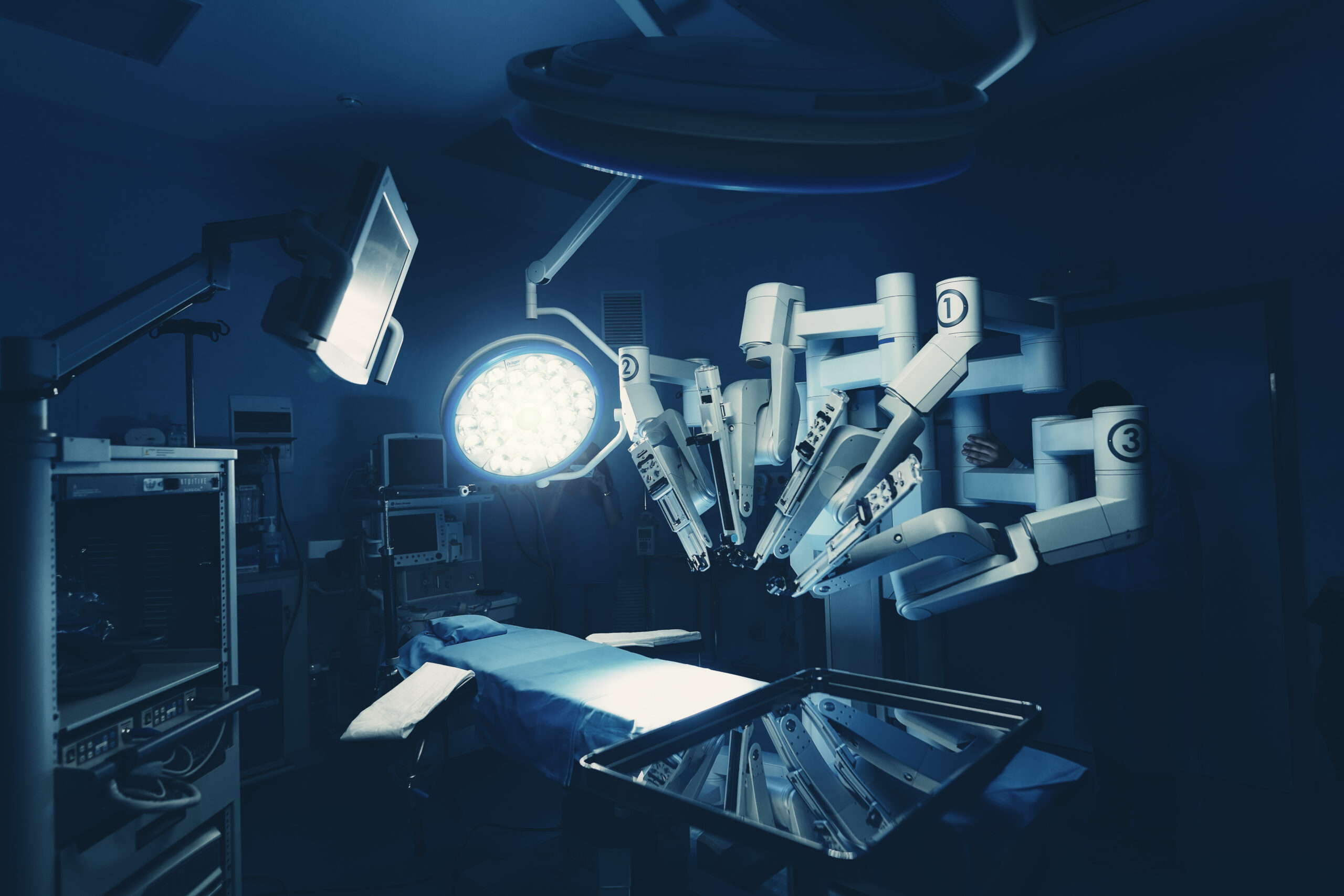
Takeaways • Robot-assisted surgery (RAS) is now an option in many specialties and for adult and pediatric patients; RAS-related ethical issues include access and patient privacy. • Intuitive Surgical continues to dominate the marketplace, but many companies are working on expanding their range of options. • Some current trends and…

As we celebrate National ASC Month, it is worth recognizing how central ambulatory surgery centers (ASCs) have become to modern healthcare. Today, there are more than 12,000 ASCs across the US, including over 6,500 Medicare-certified facilities operating some 18,800 surgical suites. With over 80% of all surgical procedures being performed…