Editor's Note Technologies continue to advance for effective, efficient, and safe surgical smoke evacuation. And healthcare organizations are reaping the rewards, including improved employee health and productivity. A surgical smoke expert recently explored the current state of regulatory, legislative, and technology developments to reduce surgical smoke exposure in Today’s Medical…
Editor's Note ASC growth continues to evolve, and over the next decade technology will significantly enable this continued growth, according to experts interviewed in a November 13 news story in Ambulatory Surgery Center News. Specifically, they noted a focus on robotics and AI to support a smooth transition for more…
Editor's Note Doctors from Scotland and the US recently completed what is thought to be a world-first stroke procedure using a remote surgical robot on a human cadaver. A professor testing the remote robotic technology at a hospital in Dundee, Scotland and a neurosurgeon in Florida conducted the thrombectomy on…

It is no long best practice to deliver education via a traditional lecture with PowerPoint slides or an unimaginative online learning module. “These techniques are boring and not effective,” says Beverly Kirchner, MSN, BSN, RN, CNOR(E), CASC, chief compliance officer for SurgeryDirect, LLC. “Staff just listen or read the information,…

Editor's Note Pairing short, human-led preoperative videos with structured postoperative texting streamlines workflows without sacrificing clinical touch at ambulatory surgery centers (ASCs), said Austin Cheng, CEO of Gramercy Surgery Center in New York City, and Tracy Hoeft-Hoffman, MSN, MBA, RN, CASC, administrator of Heartland Surgery Center in Nebraska, at the…

Editor's Note What a week it was! The 2025 OR Manager Conference just wrapped up at the Anaheim Convention Center, and perioperative leaders are already talking about what is next. Mark your calendars—the 2026 OR Manager Conference is heading to Savannah, Georgia, at the Savannah Convention Center on October 5–7,…
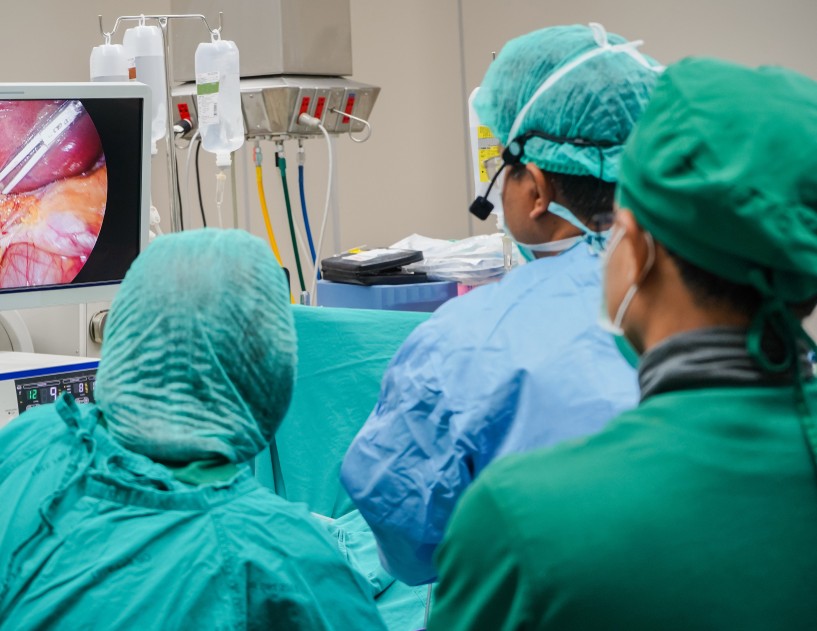
Effective communication is the foundation of patient safety in the modern surgical environment. Most surgical procedures depend on seamless collaboration among surgeons, nurses, surgical technologists, and anesthesiologists, and when communication breaks down, patient risk rises sharply. In 2024, the American College of Surgeons reported standardized surgical handoffs improved adherence to…
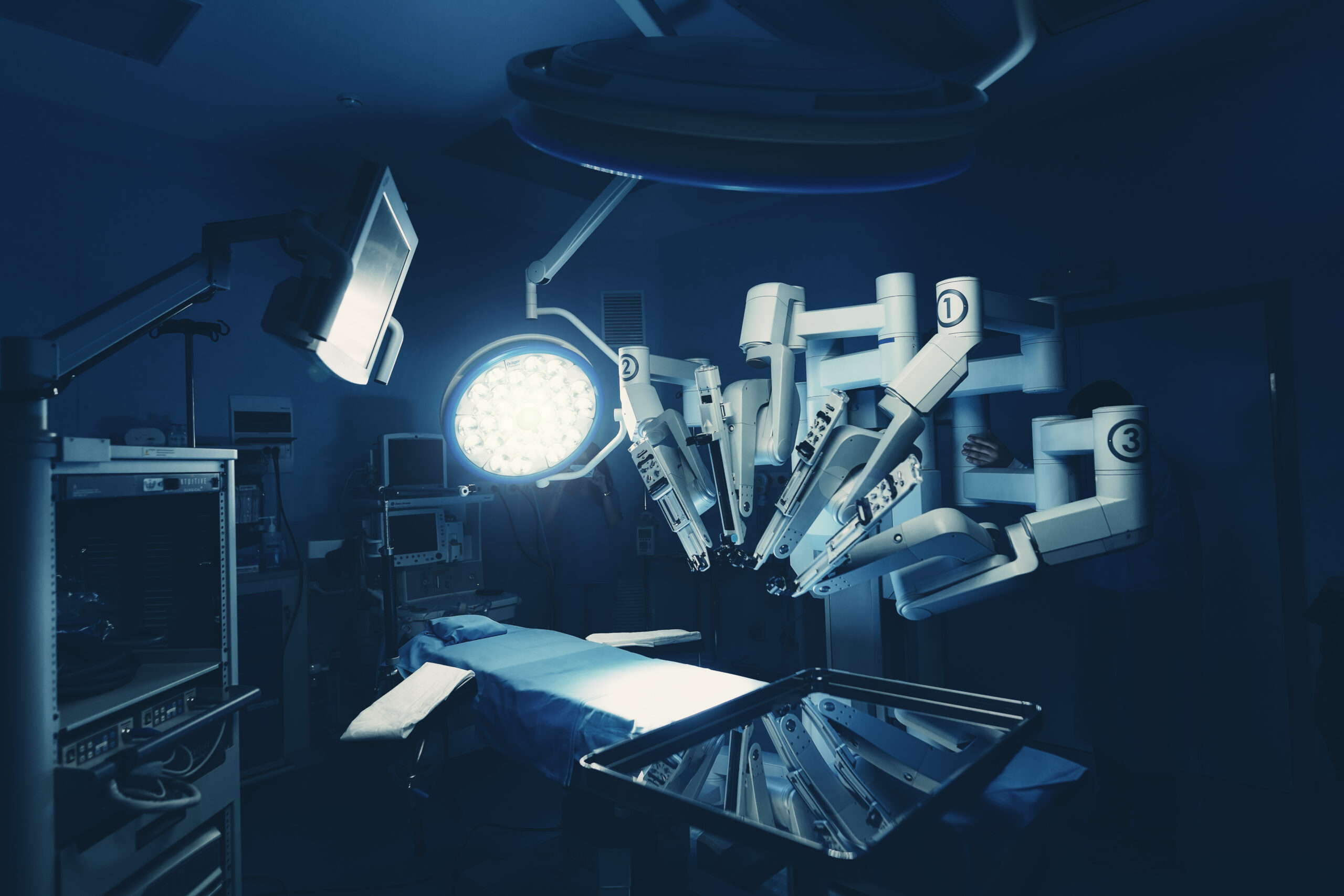
Takeaways • Determining the return on investment (ROI) for a robot-assisted surgery (RAS) program should include anticipated surgeon volume and past cases where RAS could have been used. • Some organizations dedicate staff to RAS cases, while others orient all to these procedures. • Offering ROI after hours require careful…

Artificial intelligence (AI) has made inroads into nearly every area of healthcare. With nursing shortages continuing—marked by the loss of some 100,000 nurses following the COVID-19 pandemic and projected deficits of 20% or more in some states—AI-based tools that improve access to information, streamline efficiency, monitor patients, track procedures, and…

Editor's Note Hospitals can reduce anesthesia costs by up to 30% and significantly curb provider burnout by embracing technology-enabled collaboration with anesthesiology practices, Surgical Directions August 27 reports. The report outlines how rising demand, workforce shortages, and variable pay structures have pushed anesthesia expenditures up sharply in recent years. Traditional…