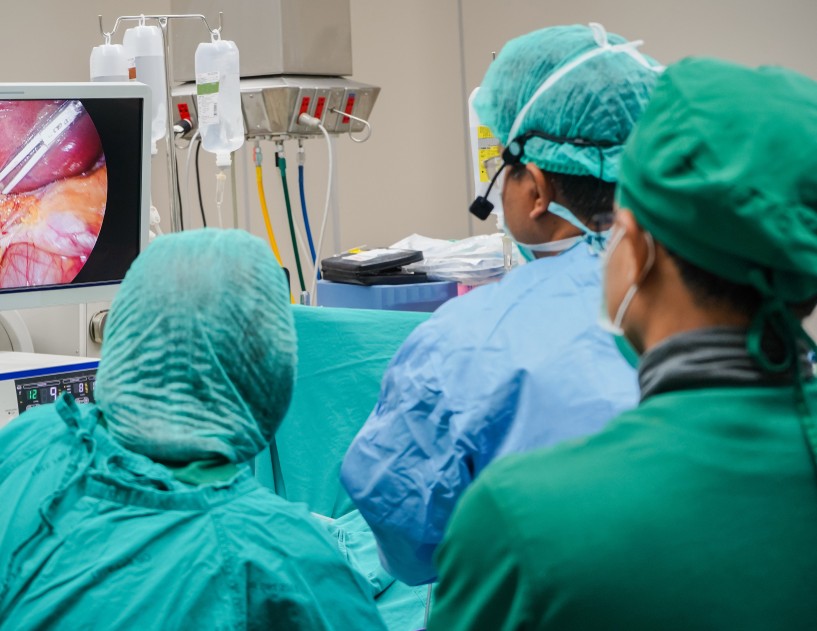
Effective communication is the foundation of patient safety in the modern surgical environment. Most surgical procedures depend on seamless collaboration among surgeons, nurses, surgical technologists, and anesthesiologists, and when communication breaks down, patient risk rises sharply. In 2024, the American College of Surgeons reported standardized surgical handoffs improved adherence to…

Editor's Note The Food and Drug Administration (FDA) on October 10 classified a cybersecurity correction involving Abiomed’s Automated Impella Controller as a Class I recall, the most serious type, according to the FDA Medical Device Recalls and Early Alerts database. While devices are not being removed from clinical settings, the…

Editor's Note The Food and Drug Administration (FDA) has issued multiple high-risk medical device recalls in recent weeks, mid-September FDA announcements report. On August 21, Medline alerted customers that some of its convenience kits contain recalled Medtronic DLP Left Heart Vent Catheters. These catheters, used in cardiopulmonary bypass, may fail…

Editor's Note The Food and Drug Administration (FDA) has issued a series of early alerts this month regarding high-risk recalls from several leading medical device makers, citing patient safety risks ranging from pump failures to vascular complications. These alerts highlight issues with products from Johnson & Johnson’s (J&J’s) Abiomed unit,…

Editor's Note The US Food and Drug Administration (FDA) issued early alerts August 6 for three medical devices due to safety concerns: Medline ReNewal’s reprocessed St. Jude Medical electrophysiology catheters, Boston Scientific’s ENDOTAK RELIANCE defibrillation leads with ePTFE-coated coils, and Boston Scientific's WATCHMAN Access Systems. The Medline alert involves specific lots…

Editor's Note Philips Respironics BiPAP A30, A40, and V30 Auto ventilators are subject to a Class 1 recall—the US Food and Drug Administration’s (FDA’s) most severe category indicating risk of serious injury or death—due to the risk for a failure in the Ventilator Inoperative alarm, which can cause therapy interruption…

Editor's Note Nearly half of hospital harm events—particularly surgical events—were not captured by reporting systems, according to a July 30 TechTarget report on new findings from the Office of Inspector General (OIG). The OIG report examined 299 harm events experienced by a nationally representative sample of 770 Medicare patients discharged…

Editor's Note A recent article from HIT Consultant highlights findings from Incredible Health’s 2025 State of US Nursing & Technicians Report, revealing mounting strain across the nursing and healthcare technician workforce. Reportedly based on insights from more than 1 million professionals, findings include: 71% of nurses report that staffing shortages…

Editor's Note The US Food and Drug Administration (FDA) has designated the recall of Ethicon Endo-Surgery, LLC’s Endopath Echelon Vascular White Reload for Advanced Placement Tip a Class I, the most severe category indicating risk of serious injury or death. As detailed in the agency’s July 25 announcement, the recall…

Editor's Note The US Food and Drug Administration (FDA) has designated a Class I recall—the most severe category indicating risk of serious injury or death—for several models of arterial cannulae manufactured by Edwards Lifesciences. Affected products include OptiSite Arterial Perfusion Cannula models OPTI16 and OPTI18, as well as Peripheral Femoral…