
Editor's Note The US Food and Drug Administration (FDA) has designated a Class I recall—the most severe category indicating risk of serious injury or death—for several models of arterial cannulae manufactured by Edwards Lifesciences. Affected products include OptiSite Arterial Perfusion Cannula models OPTI16 and OPTI18, as well as Peripheral Femoral…

Editor's Note The Department of Health and Human Services (HHS) has launched a major initiative to reform the US organ transplant system following disturbing findings about organ procurement practices. Fierce Healthcare reported the news July 21. According to the article, the initiative was triggered by a directive from HHS to…

Editor's Note The US Food and Drug Administration has designated SunMed Holdings, LLC’s recall of the Adult Manual Resuscitator with Medium Adult Mask, Bag Reservoir, Filter, Manometer and 7 ft Oxygen Tubing as Class I, the most severe category indicating serious risk of injury or death. The recall is due…

Editor's Note The Centers for Medicare & Medicaid Services (CMS) has proposed a 3.62% increase to the 2026 Medicare physician fee schedule, according to a July 14 article in Fierce Healthcare. The proposed rule sets the conversion factor at $33.42, up from $32.35 in 2025. The increase reflects a 2.5%…

Editor's Note President Donald Trump’s “One Big Beautiful Bill” could trigger more than $500 billion in Medicare cuts over the next decade unless Congress waives automatic spending rules, according to a July 9 article in Modern Healthcare. As detailed in the article, the legislation’s projected $3.4 trillion increase to the…

Editor's Note States that grant full practice authority to advanced practice registered nurses (APRNs) rank significantly higher in health system performance than those that impose physician supervision requirements, according to a July 3 report from the University of Missouri. The article focuses on a study led by researchers at the…

Editor's Note Logistical staff layoffs at the US Food and Drug Administration (FDA) are hindering the agency’s ability to scrutinize drug manufacturing safety in foreign countries, according to a July 7 report in ProPublica. A spokesperson from the US Department of Health and Human Services (HHS) told ProPublica that FDA…

Editor's Note A recent report in Becker’s Hospital Review outlines six recent examples of hospitals and health systems laying off workers in response to deepening financial strain. In a separate report, the outlet listed 11 hospitals and health systems that received credit rating downgrades from Fitch Ratings or Moody’s Investors…
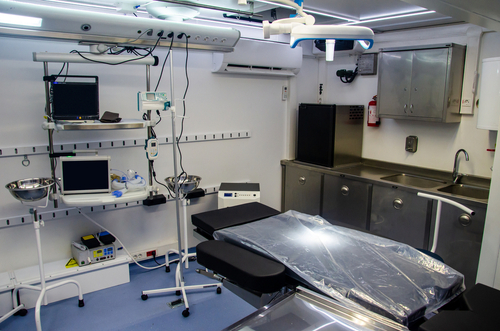
Imagine an innovative, safe, and highly efficient OR not confined by walls but on wheels—crossing rugged terrains, bustling cities, and disaster-stricken areas to deliver life-saving surgical care in underserved areas. That is the premise and promise of mobile ORs. They are not just mobile units. With some of the technological…

Editor's Note The US Food and Drug Administration (FDA) has designated Cook’s recent recall of the Beacon Tip 5.0 Fr Angiographic Catheter as a Class 1, the most severe category indicating serious risk of injury or death. The recall was reportedly motivated by reports of tip separation both prior to…