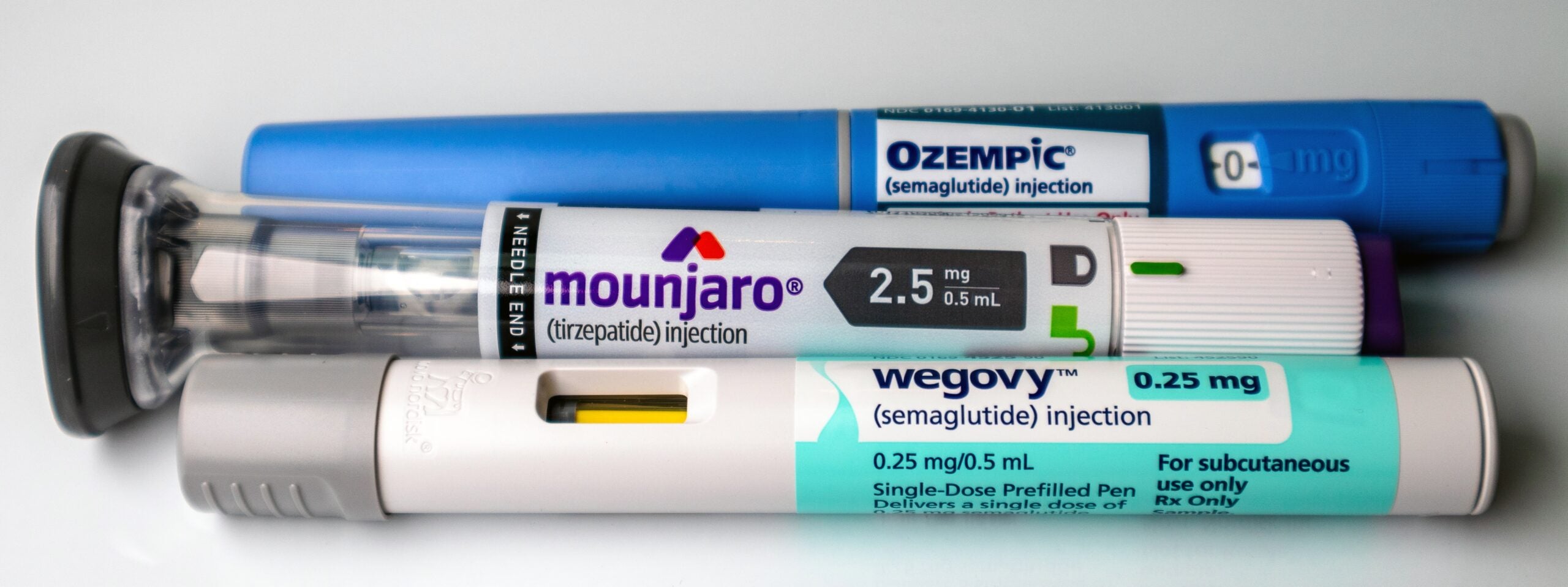
With the explosion of GLP-1–based therapies for type 2 diabetes and weight loss, perioperative nursing teams, especially in the preoperative and postanesthesia care areas, need to be fluent in recognizing these medications. Some patients may arrive on these agents—or even novel oral GLP-1s—and the physiologic effects, especially delayed gastric emptying,…

Editor's Note The Perioperative Nutrition Screen (PONS) identifies patients at greater risk of complications following pancreatic cancer surgery, according to a retrospective analysis published August 27 by Research Square. The study found that patients flagged for nutrition risk by PONS experienced longer hospital stays, higher complication rates, and were more…

Editor's Note NDM-producing carbapenem-resistant Enterobacterales are climbing fast and straining treatment choices, according to a September 23 release from the Centers for Disease Control and Prevention (CDC) and published in the Annals of Internal Medicine. The agency warns NDM-CRE infections rose more than 460% in the US from 2019 to…

Editor's Note Surgery and advanced implantable devices can give patients with drug-resistant epilepsy a far greater chance of seizure freedom than medication alone, UCSF News September 25 reports. While about 30 anti-seizure medications exist, one-third of patients do not achieve seizure control, and fewer than one in five seek care…

Editor's Note Many widely used supplements and herbal remedies can increase bleeding risk during surgery and should be stopped in advance, according to researchers at Wrocław Medical University. The findings highlight a gap in perioperative safety practices, The Am-Pol Eagle September 18 reports. The study, led by the university’s Department…

Editor's Note Sterile processing departments (SPDs) face chronic staffing shortages and underinvestment that put surgical patients at risk, according to a Surgical Directions September 18 report. It emphasizes that sterile processing technicians, who decontaminate, inspect, and sterilize every surgical instrument, remain under-recognized despite their central role in surgical safety. Per…

Editor's Note Ambulatory care leaders can now sharpen compliance strategies with the release of the 2025 Quality Roadmap from the Accreditation Association for Ambulatory Health Care (AAAHC), published September 22. The annual report distills more than a year of survey findings into practical guidance for addressing the most common accreditation…

Editor's Note Surgical quality leaders are pushing boundaries with new strategies that blend technology, frontline engagement, and national-local collaboration. That was the message from the American College of Surgeons (ACS) Quality and Safety Conference (QSC), held July 17–20, 2025, in San Diego, according to a September 10 ACS report. The…

Editor's Note Colorectal tumors once considered inoperable are now routinely treated with curative surgery, thanks to advances in multimodality therapy and complex resection techniques, Mayo Clinic September 16 reports. Decades ago, cancers invading the sacrum, pelvic organs, or major blood vessels were often deemed unresectable, leaving patients with only palliative…

Editor's Note Older age alone should not exclude patients from ambulatory general surgery. A retrospective study published in Cureus on August 27 found that patients over 75 undergoing short-stay general surgical procedures experienced complication and reintervention rates comparable to younger peers, despite higher comorbidity and anesthetic risk scores. The analysis…