
Artificial intelligence (AI) has made inroads into nearly every area of healthcare. With nursing shortages continuing—marked by the loss of some 100,000 nurses following the COVID-19 pandemic and projected deficits of 20% or more in some states—AI-based tools that improve access to information, streamline efficiency, monitor patients, track procedures, and…

Editor's Note The Food and Drug Administration (FDA) has issued four medical device recalls and early alerts between September 23 and 30, covering Automated Impella Controllers, Olympus ViziShot 2 FLEX (19G) endoscopic aspiration needles, 3M Ranger blood and irrigation fluid warming systems, and BD Alaris infusion pump sets. The notices…

Editor's Note Baxter has issued a correction notice for its Novum IQ Large Volume Pump (LVP) after identifying a serious risk of underinfusion linked to the device’s standby mode and power-off conditions. First published on April 24 on the Food and Drug Administration (FDA) website and subsequently reported by Healthcare…
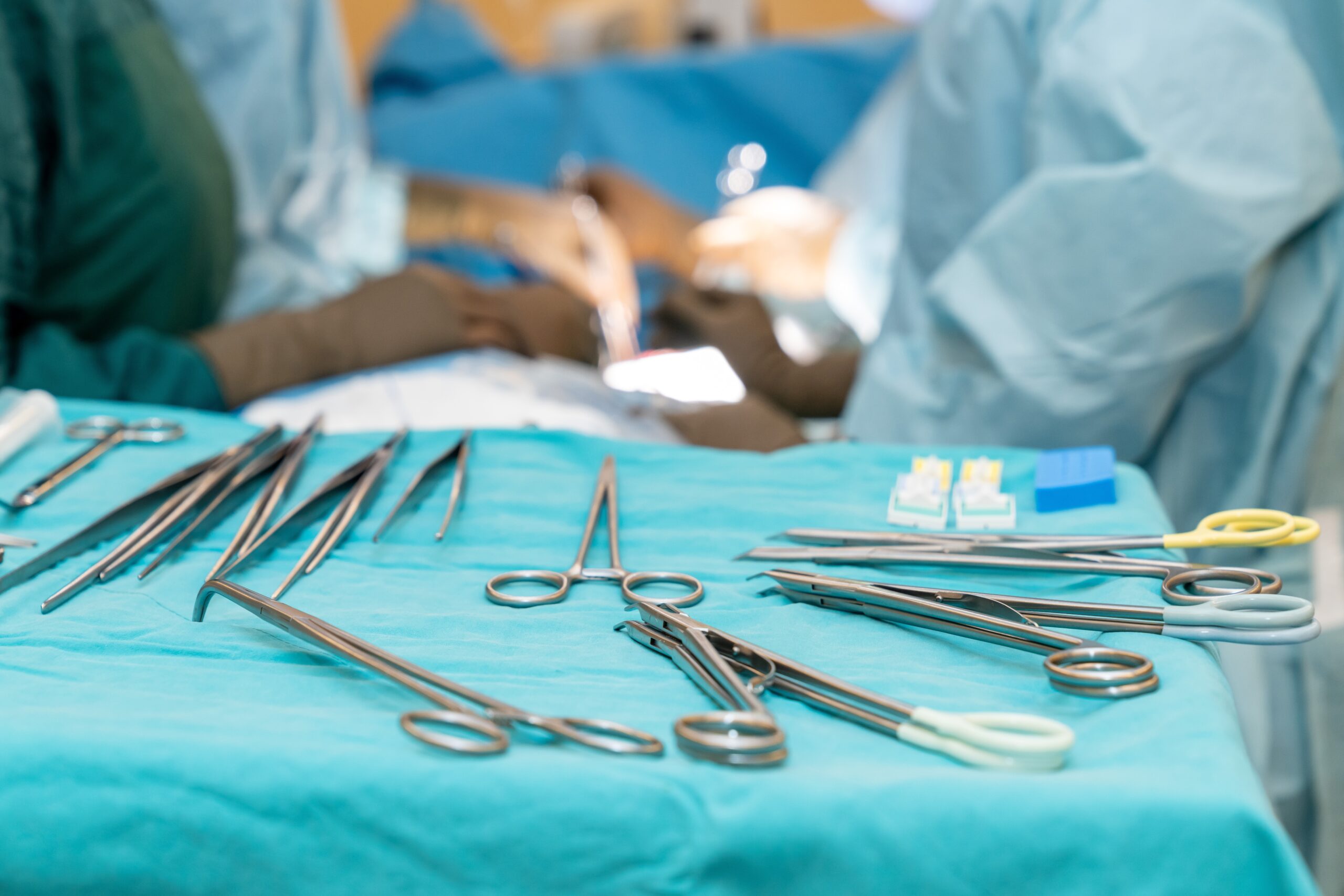
Preventing infection from contaminated surgical tools requires attention to every link in a complex chain of processes, from point-of-use pretreatment in the OR to the moment the freshly disinfected or sterilized item arrives at the next patient’s bedside. For those on the front lines, manufacturers’ written instructions for use (IFUs)…

Reliable and robust enough for daily use on most medical devices, steam is the most common sterilant in healthcare facilities. However, using steam properly requires a balancing act. For example, too much moisture can lead to wet packs, while steam that is too dry might not be sufficient to achieve…

Editor’s Note The Association for Professionals in Infection Control and Epidemiology (APIC) is advocating for clearer reprocessing instructions for medical devices to improve patient safety and efficiency, Outpatient Surgery Magazine August 19 reports. Many current instructions for use (IFUs) are considered overly complex, outdated, and difficult to interpret, especially for…