
Editor's Note COVID-19 can be considered endemic worldwide, The Centers for Disease Control and Prevention (CDC) announced on the heels of reporting a drop in overall US death rates from the disease. CDC’s classification of the disease as endemic “means, essentially, that COVID is here to stay in predictable ways,”…

Editor's Note As the Republican National Convention meets in Milwaukee to nominate Donald Trump, the party is not expected to unveil a detailed healthcare platform. However, a July 15 report in Modern Healthcare covers what plans and past records reveal about the potential direction of health policy under a GOP…

Editor's Note COVID infections are rising nationally, with cases increasing in 39 states and none showing a decline, NBC news reported June 24. The Centers for Disease Control and Prevention (CDC) tracks Covid transmission through emergency department visits, which, along with deaths and hospitalizations, have recently increased, NBC reports. California…

Editor's Note The Joint Commission is set to revise the Infection Prevention and Control (IC) chapter requirements for both office-based surgery (OBS) practices and ambulatory healthcare (AHC) organizations, effective July 1, 2025. These revisions aim to streamline the IC chapter, focusing on essential structures and processes that support quality and…
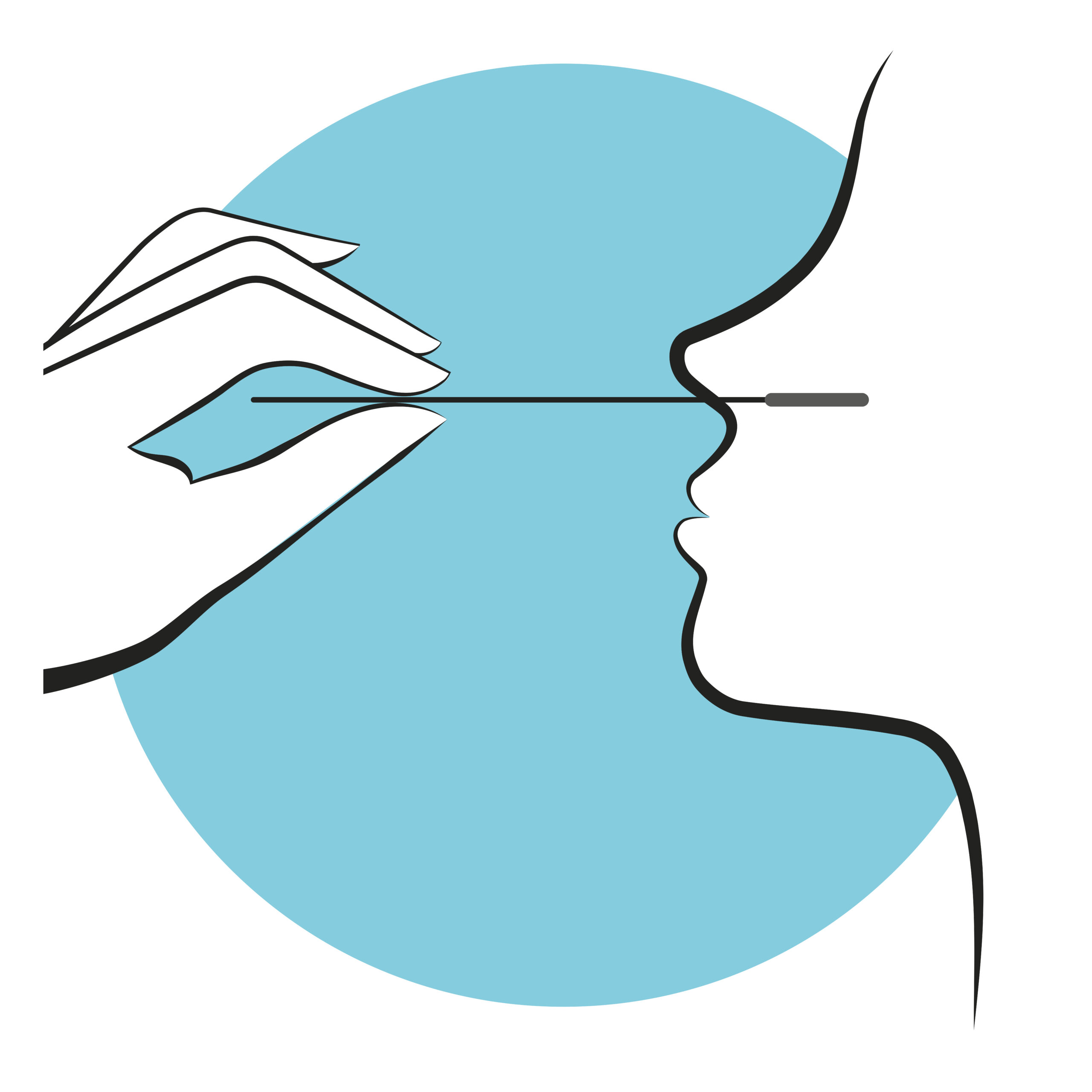
Over 20 years ago, an article from Johns Hopkins published in The New England Journal of Medicine showed that Staphylococcus aureus decolonization of the nares can decrease risk of surgical site infections (SSI). Since then, nasal decolonization—the application of a topical antimicrobial or antiseptic agent to the nares—has been adopted…

Ambulatory surgery centers (ASCs) play an increasingly crucial role in delivering outpatient surgical care that is efficient, effective, and—most importantly—safe. As the ASC sector continues to grow and evolve, maintaining best practices in hand hygiene and environmental cleaning and disinfection is imperative to protect patients from infections. ASCs also should…

Editor's Note In a move one expert calls “a complete U-turn,” the World Health Organization (WHO) has concluded that viruses transmit through primarily the air via inhalation of tiny suspended particles of saliva and mucus, KFF Health News reported on May 1. Until now, health authorities have relied on the…

Editor's Note The National Institute for Occupational Safety and Health (NIOSH), of the the Centers for Disease Control and Prevention (CDC), is releasing an innovative, hospital-tested guide aimed at addressing and mitigating healthcare worker burnout, a CDC March 18 press release reports. This initiative is a cornerstone of the Impact…

Editor's Note This spring, The Centers for Disease Control and Prevention (CDC) is expected to announce that individuals who test positive for COVID-19 no longer need to isolate if they’ve been fever-free for 24 hours and symptoms are mild or improving. Current guidelines, in place since 2021, recommend isolating for…
Editor's Note: Adherence to routine disinfection procedures may not be enough to prevent potentially harmful bacterial contamination of high-touch hospital surfaces, according to findings published January 10 in the American Journal of Infection Control. Manikins, bed rails, and workstations-on-wheels were the most contaminated surfaces. The study involved sampling and culturing…