CMS extends “Two-Midnight” rule partial enforcement delay to end of year
Editor's Note The Centers for Medicare & Medicaid Services on August 12 extended the partial enforcement delay of the “Two-Midnight” rule from September 30 to December 31, AHA News reports. The extension prohibits Recovery Audit Contractors from conducing post-payment patient status reviews for claims with admission dates October 1 to…
ACS Advocating for Critical Access Hospital Relief Act
Editor's Note A central issue discussed at the US House Committee on Ways and Means on July 28 was the Critical Access Hospital (CAH) Relief Act, HR 169, which is supported by the American College of Surgeons (ACS). Currently, for CAHs to receive Medicare Part A reimbursement, physicians must certify…
Penalties based on number of VTEs unfairly imposed
Editor's Note After a review of 128 case histories, Johns Hopkins researchers find that financial penalties imposed by federal and state agencies on Maryland hospitals based solely on the total number of patients who suffer venous thromboemboli (VTEs) fail to account for those that occur despite the consistent and proper…
Senate bill would let Medicare patients self-pay for medical devices
Editor's Note Four US Senators (two democrats, two republicans) are sponsoring legislation—The Accelerating Innovation in Medicine (AIM) Act—that would increase Medicare patients’ access to new medical devices. Currently, Medicare patients who are interested in self-paying for a device not covered by Medicare face significant administrative obstacles. Under AIM, once a…
Senate panel asking for delay of Stage 3 MU
Editor's Note The Senate Health, Education, Labor, and Pensions Committee is asking for a delay of the Centers for Medicare & Medicaid Services' Stage 3 meaningful-use rules, which providers say are costly and time-consuming. Stage 3 requires providers to send electronic summaries for 50% of patients they refer to other…
ASCA issues quality reporting alert for ASCs
Editor's Note The Ambulatory Surgery Center Association on July 7 posted a quality reporting alert that provides instructions on required reporting that all Medicare-certified ASCs must submit to the Centers for Medicare & Medicaid Services ASC Quality Reporting Program by August 15. ASCs must report on five measures (ASC-6 through…
Surgical resident work hours reform does not improve patient safety
Editor's Note Work-hour restrictions for surgical resident, revised nationally 4 years ago to protect patients against fatigue-related errors, have not had the desired effect of lowering postoperative complication rates, according to this new study. Researchers from the American College of Surgeons found no significant difference in surgical outcomes between 1…
Hospital accreditation options expand beyond Joint Commission
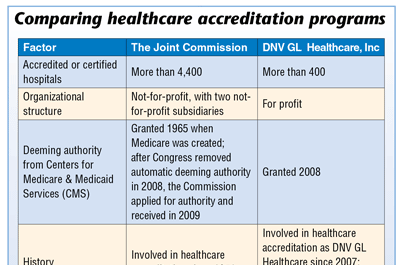
In the past, most hospitals automatically sought accreditation from the Joint Commission, but recent years have brought new players to the field, prompting hospital administrators to rethink that strategy. One relatively new player is DNV GL-Healthcare (DNV GL). Since achieving deeming authority from the Centers for Medicare & Medicaid Services…
Help is available for managing pharmaceutical waste safely and legally
Like all healthcare facilities, ambulatory surgery centers (ASCs) generate medical waste. Some of that waste is well known to be hazardous, such as blood, tissue, or used needles and syringes. Another type of waste is leftover medication, which is subject to its own regulations and disposal practices. Compliance is a…
First step taken in FDA-issued unique device identification system
Medical device manufacturers have taken the first step in complying with the 7-year unique device identification (UDI) process mandated by the Food and Drug Administration (FDA). The UDI system establishes a consistent way to label and track medical devices from production to use, and is intended to improve patient safety…

 Free Daily News
Free Daily News