 Sterilization & Disinfection
Sterilization & Disinfection
Catheter sterility concerns prompt Class 1 FDA recall for surgery trays

Editor's Note The US Food and Drug Administration has designated DeRoyal Industries’ recall of GeoMed custom tracecarts a class 1, the most serious type of recall indicating a risk of serious injury or death. According to the April 24 FDA notice, the recall is due to sterility concerns with the…
Surface disinfection: How to play your cards right with UVC light
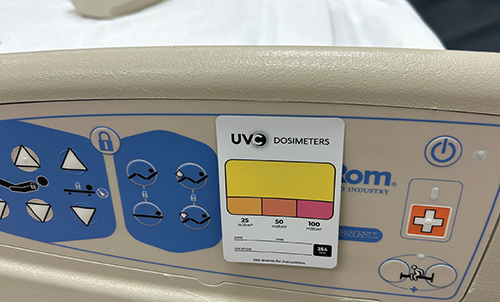
Approximately one in 31 hospital patients has at least one infection on any given day, according to the Centers for Disease Control and Prevention (CDC). In surgical settings, the risk is even higher, with up to 7% of patients developing an infection during surgery. These infections can lead to a…
EPA rule to limit dangerous emissions from medical sterilization plants

Editor's Note The Environmental Protection Agency (EPA) has finalized a rule to reduce chloroprene and ethylene oxide emissions that will impact over 200 chemical plants across the nation, including medical sterilization plants, according to an April 10 report from USA Today. Under the new rule, the EPA will cut more…
ChatGPT, Mixtral AI systems show promise in detecting healthcare-associated infections

Editor's Note Based on the performance of two specific systems in detecting healthcare-associated infections (HAIs) in a recent study, artificial intelligence (AI) could help providers enhance surveillance, streamline tasks, and free staff to focus on patient care. Published March 14 in The American Journal of Infection Control, the study assessed…
Study: UV-C light effectively disinfects non-sterile, high-touch surfaces

Editor's Note Although many studies have focused on infection transmission within the operating room, authors of research published in the March issue of the Journal of Infection Control focused their study of UV-C light disinfection on non-sterile hubs of patient care—in this case, high-touch surfaces within an academic endoscopy unit.…
Forced-air device outperforms standard endoscope drying practices, study shows

Editor's Note Authors of a recent study evaluating the effectiveness of a forced-air drying system for endoscopes argue that the results reinforce the need to re-evaluate standard drying practices. Findings were published February 24 in the American Journal of Infection Control. Wet environments resulting from inadequate drying practices can result…
Surgical scrub evolution and the future of smart medical attire

For surgeons and other medical professionals, what to wear to work is more than just an afterthought. Over the decades, surgical scrubs have undergone a significant transformation, evolving from simple, functional garments to sophisticated attire that prioritizes both comfort and infection control. They are not merely clothing but a vital…
New EPA standards to reduce ethylene oxide emissions

Editor's Note New standards from The Environmental Protection Agency promise to cut nationwide emissions of ethylene oxide—employed to sterilize more than half of US medical devices—by more than 90 percent. According to a March 15 MedPage Today report, the aim is to reduce cancer risk among the 13 to 14…
Negative pressure wound therapy reduces SSI across surgical specialties

Editor's Note Compared with standard wound dressings, single-use negative pressure wound therapy (NPWT) devices can reduce the incidence of surgical site infection (SSI) in at-risk patients with closed surgical incisions across a range of surgical specialties, according to a data review highlighted in the February issue of the American Journal…
Unveiling ECRI’s 2024 top 10 health technology hazards list

What is the purpose of the top 10 health technology hazards list, released every year by ECRI? “Our number one goal at ECRI is to reduce preventable harm,” stresses Jason Launders, MSC, former director of operations, device evaluation, at ECRI. “We know that every healthcare provider has a lot they…

 Free Daily News
Free Daily News